.
Introduction
Vitamin C, or Ascorbic acid (AA), is a nutritional factor in food that serves as an important cofactor for essential metabolic processes. Given in large quantities, AA has also been evidenced to execute favorable pharmacological activities in varied diseases.
AA is a neutral molecule with 6 carbon atoms, a ring structure with 6 oxygen, and 8 hydrogens, some of them forming hydroxyl groups with oxygen. When a hydrogen ion is lost (removed) from the hydroxyl group, the molecule becomes negatively charged and is called ascorbate. The ascorbates have a negatively charged anion associated with a positively charged cation. The anion component of the ascorbate donates electrons and is responsible for most of the biological effects. The cation can also have biological effects. Some of the ascorbate forms are sodium ascorbate, calcium ascorbate, magnesium ascorbate, potassium ascorbate, manganese ascorbate, and zinc ascorbate (Figure 1).
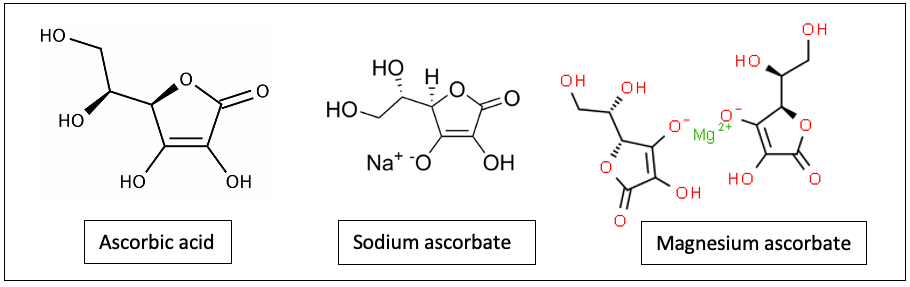
Figure 1. Ascorbic acid (AA), sodium ascorbate and magnesium ascorbate
AA can be ingested in the form of ascorbic acid ascorbate. Regardless of the form received by the body, it will interact with other molecules and membranes in a physiological dance that will transform one form into another through perpetual cycles of simple oxidation-reduction reactions (RedOx) or complex conformational changes in DNA (Yoshikawa et al., 2003). This AA molecule’s versatility is important for the process of photosynthesis and mitochondrial ATP production (Gonzalez et al., 2010).
Research has demonstrated that AA is exceptionally safe in humans at very high doses and has relevant pharmacological and physiological pleiotropic effects (Padayatty et al., 2010; Magrì et al., 2020; Luchtel et al., 2020; Guo et al., 2022; Lee et al., 2019; Ried et al., 2016; Vollbracht et al., 2018; Emadi et al., 2019; Doseděl et al., 2021; Carr and Maggini 2017). These effects explain its wide array of clinical uses that improve patient outcomes in burns (McGregor & Biesalski, 2006), infections (Schencking et al., 2012; Chen et al., 2019; Gonzalez et al., 2018; Marcial-Vega et al., 2017; Mikirova et al., 2014), sepsis, and many other conditions (Holford et al., 2018; Fowler et al., 2019; Mahmoodpoor, 2021; Schencking et al., 2012). Despite its proven importance in physiological and pharmacological processes, most clinical curriculums fail to teach the importance of assessing ascorbate sufficiency (Mandl et al., 2009).
AA is a cofactor in hydroxylation reactions in the formation of various neurotransmitters, hormones, and in collagen stabilization, the protein that comprises 30% of human cellular protein mass (Ballaz et al., 2019; Gallie et al., 2013; Pandipati et al., 1998; Kishimoto et al., 2013). Ascorbate is also fundamental in RedOx homeostasis in the mitochondria and endoplasmic reticulum (Boel et al., 2019; Pozzer et al., 2021; Singh-Mallah et al.,. 2019). The RedOx potential of AA has a central role in the regeneration of vitamins within the body, such as alpha-tocopherol (Niki, 1987) and ubiquinone (Beyer, 1994). In addition, AA also Is involved in epigenetic regulation of genomic stability and has modulatory effects on nucleic acids and histones with implications for carcinogenesis and other relevant biological processes (Young, 2015, Brabson et al., 2021).
This article will discuss the physiological effects and response of the human body to a spectrum of concentrations of AA ranging from pathologically low to pharmacologically high and proposes a novel guide to its interpretation.
Physiologic Ascorbic Acid
The Human Metabolic Disadvantage in Ascorbic Synthesis
Contrary to humans, a vast majority of vertebrates can synthesize AA in accordance with physiologic demands. Typically, they convert D-glucose to AA through a biochemical pathway mediated by the enzyme l-gulono-g-lactone oxidase (GLO), which catalyzes the last step of AA biosynthesis. When sufficient AA is present, it helps control excess inflammation, support leukocyte function, inhibit microbial pathogen growth and neutralize harmful reactive oxygen species (ROS). However, humans lack a functional gene and, therefore, the GLO enzyme (Nishikimi et al., 1991). Homo sapiens are not able to synthesize AA and acquire the necessary amounts of this molecule by administering nutritional or pharmacologic doses, according to the current physiological demands.
AA Absorption and Plasma Concentrations in Health and Disease
In its reduced form, AA has a high electron-donating potential. Once oxidized to dehydroascorbic (DHAA) acid it may be converted back to the active reduced form. This gives the molecule the capacity to neutralize excess damaging reactive oxygen species and serve to provide stores/transports of the cell metabolic antioxidant potential (Cite). AA concentrations in body fluids and tissues are largely regulated through absorption, tissue accumulation, utilization, and renal reabsorption.
Inter-individual differences in genetics, metabolism, physiology, absorption, activity level, and body size affect the optimal amount of AA needed to maintain health. An individual’s AA needs may also vary depending on the conditions that create an intense change in physiology such as (Long et al., 2003), infection (Tanzer, 1993; Li et al., 2006), as well as exposure to certain substances, all of which create excess oxidative stress, inflammation, and other increased metabolic demands. The utilization of AA increases during stress-inducing experiences, such as surgery Fukushima & Yamazaki, 2010), diabetes (Pecoraro & Chen, 1987), critically ill (Berger, 2015) patients after severe burns (McGregor & Biesalski, 2006), exposure to tobacco (Preston, 2006), and cancer (Mayland, 2005).
Pharmacokinetics of Ascorbic Acid
Absorption and Distribution
The absorption of AA is dose-dependent. Low, single oral doses of 30–180 mg/day are approximately 70-90% absorbed. As single oral doses approach 1 g/day and higher, absorption falls to less than 50% and unmetabolized AA is excreted in the urine (Jacob, 2002).
The absorption of reduced AA or dehydroascorbic acid (DHAA oxidized form) in the intestinal membrane can occur via a family of sodium-dependent AA active transporters (SVCT) or through facilitated diffusion via Glucose (hexose) transporters (GLUT1 or GLUT3 transporters), respectively (Lykkesfeldt, 2019). The pH level in the intestine is also a regulatory mechanism of AA absorption (Sobala, 1989). The pharmacokinetics of AA is highly regulated by the transporters SVCT. Because there tSVCT1 is responsible for in whole-body dynamics of AA, while SVCT2 activity provides protection against oxidative stress in metabolically active cells (Savini 2008). There are many types of SVCTs distributed in the body and these can be tissue-specific. Therefore, the metabolic activity of cells and the density membrane transporters in a tissue facilitate the accumulation and storage of AA in tissues depending on primary need or susceptibility to depletion. This means that the distribution pattern of AA differs between and within organs and tissues. For example, the normal concentration of AA can vary from 0.2 mM in the muscle and heart, and up to 10 mM in the brain and adrenal gland (Lykkesfeldt &Tveden-Nyborg, 2019).
In lower physiological concentrations, AA time concentration curve follows a linear dose-dependent or constant (zero-order) behavior.However, at higher pharmacological concentrations it follows first-order pharmacokinetics, which means that at higher concentrations, the higher the clearance. Thus, the aggregate half-life of AA at pharmacologic concentration is about 2 hours after infusions 50 grams and higher and volume of distribution 0.19 L/kg (Padayatty, 2004).
This was observed in studies in patients with advanced cancers using 60 grams of intravenous (IV) AA (Nielsen, 2015; Stephenson, 2013). In a recent study in critically ill patients with septic shock, using 1.5 gm IV every 6 hours, AA volume of distribution was 23.3 L, and the half-life 4.3 h (Hudson, 2019). Although in these studies the authors have reported biological half-lives of AA that ranged from 2-4.3 hours/ This is likely an aggregate effect of tissue AA redistribution. Therefore, the actual elimination half-life of AA seems to be shorter (approximately 30 minutes) following a rapid IV administration, as can be seen in Figure 2 of the publication discussing AA Pharmacokinetics and its implications for oral and intravenous use (Padayatty et al., 2004)
Metabolism
AA takes part in a myriad of physiological reactions as a cofactor or as an electron donor. The reduced form AA donates two electrons to produce the oxidized form (DHA) serving its antioxidant function.
AA itself is oxidized to an intermediate, ascorbyl free radical, which at the systemic can convert to AA and DHA. DHA’s biological half-life is a few minutes brief (Bode, 1990) because it is efficiently reduced intracellularly by a variety of cell types. DHA is generally reduced back to AA by enzymatic means, an efficient intracellular process in healthy individuals. However, smoking and disease states increase the turnover of AA requiring more intake to meet physiologic demands (cite).
Turnover of AA is associated with the catabolism of DHA, starting with hydrolysis through a series of enzymes with products entering the pentose phosphate pathway for further degradation (Banhegyi, 1997).
Excretion
AA is quickly eliminated through glomerular filtration with no significant reuptake. Following an intravenous high-dose AA, a biological half-life of about 2 hours has been reported (Padayatty, 2004). However, based on prior data from Levine and Padayatti, the actual elimination half-life of AA could be shorter than that (approximately 30 minutes) following a rapid IV administration (add citation date) [Padayatty, 2004]. The apparent discrepancy appears to come from the fact that biological half-live estimations do not rid of the impact that some concomitant events (e.g., distribution delays, lag time) have on drug disposition kinetics as the method to estimate the elimination half-life does (Nerella, 1993).
Therefore, it is expected that after achieving millimolar plasma concentrations by intravenous infusion, blood plasma levels are normalized to physiological levels in approximately 16 hours. However, disease states may alter excretion dynamics. Animal data support the hypothesis that tumor tissues maintain an elevated level for as much as 48 h (Campbell, 2016). This could be caused by increased tissue uptake related to metabolic use and increased tumor GLUT expression (cite) [Blaszczak et al 2019]. Increased tumor ascorbate was associated with slowed tumor growth, reduced tumor microvessel density, and decreased hypoxia. The hypoxic tumor environment does not appear to causally affect AA concentration.
Genetic Polymorphisms
Genetic polymorphism refers to allelic variations which alter the DNA sequence at a given locus. This variation can result in changes in proteins that may have functional or structural implications, such as the reduced affinity of an enzyme for its cofactor (Ames, 2004).
SVCT (sodium-dependent vitamin C transporters) are involved in the tissue distribution of AA. Thus, SVCT variants can result in reduced AA saturation in a specific tissue (Michels, 2013). Additionally, genetic variants of proteins can suppress oxidative stress or detoxify damaged biomolecules. The antioxidant enzyme glutathione peroxidase (GPx1) has an important role in determining the oxidative stress of individuals. It has been found that individuals with a less active genetic variant (GPx1 rs1800668 genotype) produce significantly less total glutathione, reduced/oxidized glutathione, and ubiquinone when compared to healthy individuals (Gugliandolo et al. 2016). Patients with more active glutathione or taking glutathione supplements will reduce oxidative stress; those that have a less active variant or are exposed to chemicals that produce oxidative stress. These effects have an impact on AA use and therefore its concentration level in the human body. The higher the level of reducing agents the lower the oxidative stress and therefore the lower the consumption of AA in redox functions.
Genetic variants in HP (Human plasma haptoglobin) GST, (glutathione-s-transferase) and SOD2 (superoxide dismutase) have known roles in oxidative stress (cite) (Sitar et al. 2013, Levy et al. 2010, Pintér et al. 2017, Manivasagam et al. 2020). Single nucleotide polymorphisms in each of these genes were found to be related to AA status. This suggests that genetic variations of other antioxidant-related genes could alter AA by utilizing less AA in redox activity. In summary, here is an indication that genetic alterations, in the form of single-nucleotide polymorphisms, gene duplications, or gene deletions, alter AA levels in the human body (Michels, 2013).
Epigenetic reprogramming in cancer cells involves DNA hypermethylation and histone modification (Yun, 2012). It has been found that TET (Ten-eleven Translocase) proteins can be activated by the AA as a cofactor. TET proteins are enzymes that can demethylate DNA. Neomorphic mutations of Isocitrate dehydrogenase expression can produce reduced TET activity increasing DNA methylation and promoting the expression of tumor-associated genes (Lu et al., 2012). In some lymphomas, AA enhanced mutated TET activity, leading to DNA demethylation, increased expression of tumor suppressor genes, and chemosensitivity (Shenoy, 2017).
Emerging evidence has suggested that the epigenetic mechanisms by which AA may enhance gene reprogramming in somatic cells are due to its cofactor role in Fe (II) and 2-oxoglutarate-dependent dioxygenases, including the TET and histone demethylases (Kuiper et al., 2014). Recently, Liu et al. examined the available evidence concerning the postulated role of AA in DNA and histone demethylation and highlighted its potential involvement in regulating N6-methyladenosine demethylation (2021). Liu et al. also indicated an affiliation of demethylases with AA-facilitated epigenetic reprogramming and a contribution of AA to epigenetic regulation (2021).
Prior studies have also shown that AA administered at high intravenous doses can suppress cancer cell growth through epigenetic mechanisms, namely DNA demethylation (Mastrangelo et al., 2018). Steers et al. have proposed that the co-administration of high IV-AA doses and DNA methyltransferase inhibitors may offer a therapeutic advantage in the treatment of pancreatic cancer through both direct cytotoxic mechanisms and epigenetic alterations (2021).
Toxins and Disease
Toxins, injury and disease create oxidative stress in various tissues, subsequently increasing AA body utilization and depletion if AA consumption is inadequate.
Tobacco smoke is a toxic substance consumed by humans that significantly impact AA dynamics Research has demonstrated that active smoking typically diminishes AA plasma by 25–50% (Lykkesfeldt, 2006), while passive tobacco smoke exposure reduces plasma AA concentrations by approximately 12-25% (Preston, 2006).
It has been proposed that critically ill patients can tolerate higher/ supratherapeutic doses of orally ingested AA without experiencing significant gastrointestinal upset. This method was coined “titrating to bowel tolerance”. Cathcart reported that at least 80% of adult patients will tolerate 10 to 15 grams of AA per day without having diarrhea when AA was dissolved in water and given in divided doses (1981). The absorbed dose is proportional to the severity of the illness with intakes over 100 grams being tolerated. In the case of very toxic diseases, doses may have to be taken every half hour. Short delays in taking these doses may prolong the disease (Cathcart, 1981).
Absorption and distribution of AA into the diseased tissues occur at an accelerated rate, presumably because of increased AA metabolism. More specifically, it is a change in signaling and controls that open up transport channels. Therefore, to supply the metabolic demand, this frequent dosing provides an adequate amount at an adequate rate (Cathcart 1981). Some conditions known to be associated with low levels of AA include cancer (Mayland 2005; Fritz et al., 2014), and viral illnesses (Tomasa-Irriguible & Bielsa-Berrocal, 2021), sepsis (Marik, 2018), and diabetes (Ali et al., 1989). Previously, a study had demonstrated that plasma total AA was significantly lower in individuals with diabetes compared to age and sex-match controls (N=100). In addition, in patients with diabetes and diabetic retinopathy, the plasma total AA was significantly lower than that of uncomplicated diabetics (Ali et al., 1989). Another controlled study showed that low AA levels in diabetes appear to be a consequence of the disease itself and not due to inadequate dietary intake of AA (Sinclair, 1994). Furthermore, the presence of complications seems to be an important prognostic factor in AA depletion. For example, diabetic patients with microangiopathy have lower levels of AA than age-matched diabetics without microangiopathy (Sinclair, 1991).
Cancer, Inflammation, and Bacterial/Viral Infection
A study of 50 patients recruited from a large hospice with advanced cancer from different types (i.e., brain, breast, bronchial, urogenital, gastrointestinal, prostate, etc.) found that 30% of individuals were deficient (<11 μM) and 42% were low (11.1 -23 μM) in plasma AA. Additionally, low plasma AA was found to be significantly associated with low albumin, low PLT, high CRP, and shorter survival (Mayland,2005).
AA at high doses (7,500 and over mg/day), especially when producing high micromolar or millimolar concentrations has been shown to exhibit anticancer (antineoplastic), anti-inflammatory, antioxidant, immunomodulatory, and antiviral effects among others (Levine et al., 2011; Sun et al., 2019; Nakajima et al., 2019; Cheng et al., 2012; Kim et al., 2013; Sorice et al., 2014; Feigen et al., 1982). There are many documented anticancer mechanisms described for AA, most notably, the preferential promotion of hydrogen peroxide and oxidative stress in cancer cells, AA-mediated downregulation of HIF transcriptional activity, and AA-regulation of epigenetic changes such as DNA demethylation. This DNA methylation process is facilitated by the ten-eleven translocation enzyme activation which results in the re-expression of tumor suppressor genes in cancer cells (Vissers & Das, 2018).
Intravenous AA at doses of 7.5-50 g can reduce inflammation by as much as 44%, as measured by C-reactive protein or CRP (Mikirova et al., 2012). Several studies describe the various mechanisms by which AA enhances the function of leukocytes. These include chemokinesis and chemotaxis (Schwager et al., 2015), phagocytosis (Shilotri, 1977), the production of lysosomal enzymes (Anderson 1982), the generation of reactive oxygen species (Sharma et al., 2004), microbial killing (Vilchèze et al., 2018), up-regulation of the antibody response (Mitsuzumi et al., 1998), and increased interferon production (Stone, 1980).
In addition, studies have shown many clinical benefits, including lowering infection risk (Vorilhon et al., 2019; Kim et al., 2018). Studies in septic mice suggest that an rate in septic mice occurs by activating Nrf2/HO-1 signals (Kim et al., 2015).
In-vitro observations with pharmacologic concentrations of AA (millimolar range) suggest a direct antiviral effect (Furuya et al., 2008; Shatzer et al., 2013), consistent with clinical observations of patients with Epstein-Barr viral (EBV) infection (cite). Moreover, intravenous AA has demonstrated clinical benefits against different viral infections, including SARS-Cov-2 (Schencking et al., 2012; Chen et al., 2019; Gonzalez et al., 2018; Marcial-Vega et al., 2017).
The use of AA as an effective antiviral has been documented as early as 1949 when Frederick R. Klenner reported the ability of AA to potentially cure many different acute infectious diseases and to neutralize toxins (Klenner, 1949; Klenner 1971). The caveat was that the AA needs to be provided in sufficient doses, repeated at short intervals, and continued for a long enough period. Klenner claimed that AA is a powerful oxidizer and when given in massive amounts such as 50 grams to 150 grams, intravenously (Klenner, 1971). Klenner’s report detailed that supratherapeutic doses (1000 -2000 mg) of AA, administered orally or intramuscularly led to the resolution of poliomyelitis in 60/60 (100%) patients (Klenner 1949). He also reported the cure of advanced polio and its associated flaccid paralysis with AA in 1951. Other clinicians supported Klenner’s reports of AA’s therapeutic effect on polio (Greer, 1955; Baur, 1952).
These results are consistent with previous in-vitro and in-vivo research that has shown that AA inactivates polio, herpes, vaccinia (Kligler, 1937; Turner, 1964), tobacco mosaic (Lojkin, 1936), bacteriophage (Lominski,1936; Murata, 1975; Morgan, 1976; Richter, 1982), enteroviruses (Salo, 1978), influenza (Cheng, 2012; Chen, 2014), and rabies (Amato, 1937) viruses.
Intravenous AA administration has been successfully used (complete clinical recovery) in the treatment of viral encephalitis (Klenner, 1949; Klenner, 1951, Klenner 1953; Klenner, 1971), viral pneumonia and bronchitis (Dalton 1962), measles (Joffe, 1983), mumps (Karam, 1953), Herpes (Zureick, 1950), influenza (Cai, 2015) and rabies in guinea pigs (Banic,1975). Human case reports have also supported that the intravenous administration of AA is useful in the treatment of influenza (Vilchèze et al., 2018) mononucleosis (Mikir ova, 2014), chikungunya (Marcial-Vega, 2015, Adrover, 2015), Zika (Gonzalez, 2016), and SARS COV-2 (Gonzalez, 2020).
Intravenous Administration
Klenner’s extensive work with intravenous AA infusions in 1949 significantly influenced the development of the Riordan Clinic, founded in 1975 (under the name The Center for the Improvement of Human Functioning). By 2015, Riordan Clinic had delivered over 70,000 infusions (in a period of 40 years) with a low frequency of mild to moderate, and usually transient, side effects (Riordan Clinic, 2015). This is consistent with the study of FDA’s Adverse Events Database and a survey of 172 practitioners who administered IV-AA to 11,233 patients in 2006 and 8,876 patients in 2008. The average dose was 28 grams every 4 days, with 22 total treatments per patient. Adverse events were reported in 101 patients, including lethargy/fatigue in 59 patients, change in mental status in 21 patients, and vein irritation or phlebitis in 6 patients (Padayatty et al., 2010).
High-dose intravenous AA (HDIVAA) has been used as therapy for a variety of conditions ranging from infectious diseases of bacterial and viral origin to adjuvant therapy for cancer, and many others. A clinical protocol developed over the past several decades utilizing HDIVAA summarizes principles of treatment, rationale, baseline workup, infusion protocol, precautions, and side effects (Riordan et al., 2003).
Precautions
Renal function and hydration – A prospective study of 157 patients receiving intravenous vitamin c supplementation (IVC) determined that IVC was not clearly associated with patient-reported renal stones (Prier et al 2018). Adequate renal function, hydration, and urine voiding capacity must be documented prior to starting high-dose IVC therapy. Calcium oxalate stones during or following IVC are rare (Riordan et al., 2005). In a later study conducted in a group of 16 healthy individuals with normal renal function, intravenous doses ranging from 0.2 to 1.5 g/kg body weight less
G6PD – Hemolysis has been reported in patients with glucose-6-phosphate-dehydrogenase (G6PD) deficiency when given a high dose of intravenous AA (Campbell et al., 1975). Therefore, an assessment of the G6PD level is necessary before beginning IVC.
Transient electrolyte disturbance – Due to the chelating effect of IVC, some patients may complain of shakiness due to low calcium or magnesium. An additional 1.0 mL of MgCl added to the IVC solution will usually resolve this. If severe, it can be treated with an IV push of 10 mL of calcium gluconate, 1.0 mL per minute (Riordan et al., 2003).
Venous irritation – IV irritation may occur at the infusion site. This can be caused by an infusion rate exceeding 1.0 gram/minute. The protocol suggests adding magnesium to reduce the incidence of vein irritation and spasm (cite) (Riordan et al. 2003).
Osmolarity and pH – To facilitate comfortable infusion, in addition to infusion rate and other additives previously mentioned, osmolarity and pH are important factors. Osmolarity refers to the concentration of the solute or the number of solute particles per 1 L of solvent. The pH is the concentration of hydrogen ions, H+, in a solution. Human studies of osmolarity-induced phlebitis have arrived at different conclusions, but the most often cited reference found the lowest risk of phlebitis occurred with solution osmolarities under 450 mOsm/L, moderate risk at 450 to 600 mOsm/L, and the highest risk over 600 mOsm/L (Gazitua et al 1979). Human trials measuring the impact of pH on peripheral veins found that neutralizing the pH to 7 – 7.4 significantly reduced the incidence of phlebitis (Eremin &Marshall 1977; Fujita et al 2000)
Table 1 lists the calculated osmolality of various amounts of fluid volume. Our experience has found that osmolality of less than 1200 mOsm/kg H2O is tolerated by most patients. A low infusion rate (0.5 grams IVC per minute) also reduces the tonicity, although up to 1.0 grams per minute can be used in order to achieve higher post IVC saturation levels. (Pre and post serum osmolality measurements are advisable at this dose as per the Riordan Protocol (Riordan et al. 2003).
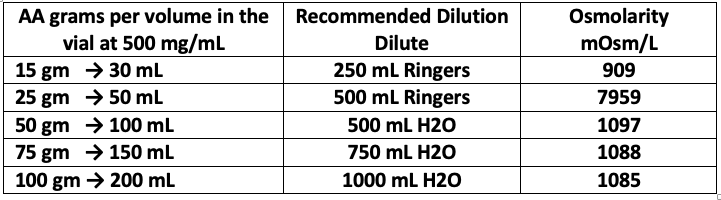
Table 1. Recommended Dilution and Osmolarity
The Need for New Guidelines
Since humans cannot synthesize AA, they are dependent on dietary intake and or supplementation. Contrary to expectations, vitamin insufficiency is common even in high-income countries. Since AA demands increase during stress, it is often depleted in patients with varied conditions, Understanding AA plasma concentration could be a useful tool for patient assessment and monitoring. The amount of AA needed to prevent acute scurvy is small and believed to be obtained in Western diets. However, the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES), indicated that the prevalence of low plasma AA concentrations (insufficiency) is as high as 22% to 33%, with 7% to 14% of people showing scorbutogenic deficiency (Schleicher, 2009; Cahill, 2009). However, data from subsequent National Health and Nutrition Examination Survey (NHANES) 2005-2016, revealed an increased prevalence of insufficient AA (inadequate) intake of 46% (Carroll, 2020). Studies in India, Malaysia, and China demonstrate similar or higher deficiencies of AA(Hughes, 1999). In Mexico,23% of children and 39% of women present with vitamin C deficiency (Villalpando, 2003). More recently, AA insufficiency among healthy people in the USA was reported to be 45% (Reider et al, 2020). Given these statistics, it may be presumed that the vast majority of people with certain risk factors or patients with acute or chronic conditions are depleted of this vitamin, which makes them more vulnerable to slow recovery or suboptimal clinical outcomes.
Ingestion, Dosing, and Plasma Concentrations
Scurvy
Scurvy has been defined as a collection of symptoms related to deficient AA in the body (anemia, myalgia, edema, petechiae, gingivitis, poor wound healing, and others). These symptoms are associated with plasma AA levels below 1.5 mg/L (0.0085 mM/L, 8.5 µM/L) (Hage 2018) or below 1.9 mg/L [0.011 mM (11 µM/L)] [The unit formats µM µmol/L etc are varying I suggest µM/L for consistency] (Nyyssönen 1997; Food and Nutrition Board, 2000).
Marginal Hypovitaminosis
Marginal hypovitaminosis or low plasma levels is a state of minimal reserves which can lead to scurvy. Hypovitaminosis is characterized by AA concentrations below 23 µM/L (Smith, 1987; Carr, 2016; Carr, 2016; Jacob, 2002). It is assumed that adequate AA levels depending on the criteria, is likely anything above 23 µmol/L. More generously, as recommended by a group of European Countries, about 50 µM/L can potentially compensate for some normal metabolic losses (Krajcovicova-Kudlackova, 2007; Brubacher, 2000, EFSA NDA Panel, 2000). Consumption of 5-to 9 servings of fruits and vegetables daily or a 200 mg AA supplement has been estimated to produce near steady-state AA plasma concentrations of 70-80 μmol/L (Levine, 1996).
Oral Ingestion
Vigorous oral ingestion results in peak values that reportedly do not exceed 220 μmol/L in healthy volunteers (Padayatty, 2004). The dynamic flow model proposes restoring human physiology to nearly that of animals that synthesize their own AA. The mean and minimum plasma levels in dynamic flow are consistent levels of about 220 μM (Hickey, 2005).
Intravenous
Only when AA is given intravenously in multi-gram doses can a supraphysiological (millimolar) concentration can be achieved. A supraphysiological concentration of AA has been reported to have important pharmacologic properties and a significant impact on patient outcome (González, 2002; Verrax, 2009; Riordan, 2004; Chen, 2005; Takahashi, 2012; Raymond, 2016; Ma, 2014). Doses around 1.5 mg/Kg and up to 100 gm of intravenous AA have been shown to produce concentrations between 25-30 mM/L (Hoffer et al., 2008; Monti, 2012).
A New Optimal Concentration and Dosing Scheme
Nearly 20 years ago, a previous guide for interpretation of plasma AA interpretation by Jacob and Sotoudeh (2002) proposed 3 levels: adequate (>23 µM), low (23 – 11.4 µM), and deficient (<11.4 µM) (2002). Levine et al. (1996) suggest 70-80 microM/L as a safe level when making dietary allowance recommendations. Despite being valuable, this guideline omits concentrations achieved when patients are receiving a clinically relevant range of oral doses and intravenous doses of AA. In our proposed table we include two levels of oral supplementation and two levels of intravenous dosing to serve as a guide for clinical decisions. This guide includes some physiological or pharmacological effects, dosing, and range in concentrations in both mg/L and µM units. However, chronic conditions such as cancer and diabetes, toxins, and trauma can be an important and dynamic source of AA turnover.
At this time, there is insufficient evidence to determine the optimal concentration and dosing regimen for each condition. A patient with a serious infection, cancer, or trauma might need different frequencies and the optimal concentration might vary according to severity, comorbidities, and other factors. In the case of cancer, it is thought that effective distribution of AA is necessary throughout the tumor environment. (Vissers & Das, 2018).
A dosing regimen that is smaller in magnitude and more frequent will produce less fluctuation in AA plasma concentration but may be more difficult to achieve than single high dose administration. For the most severe cases, the preponderance of the data so far supports that robust intravenous doses are necessary to produce the best results.
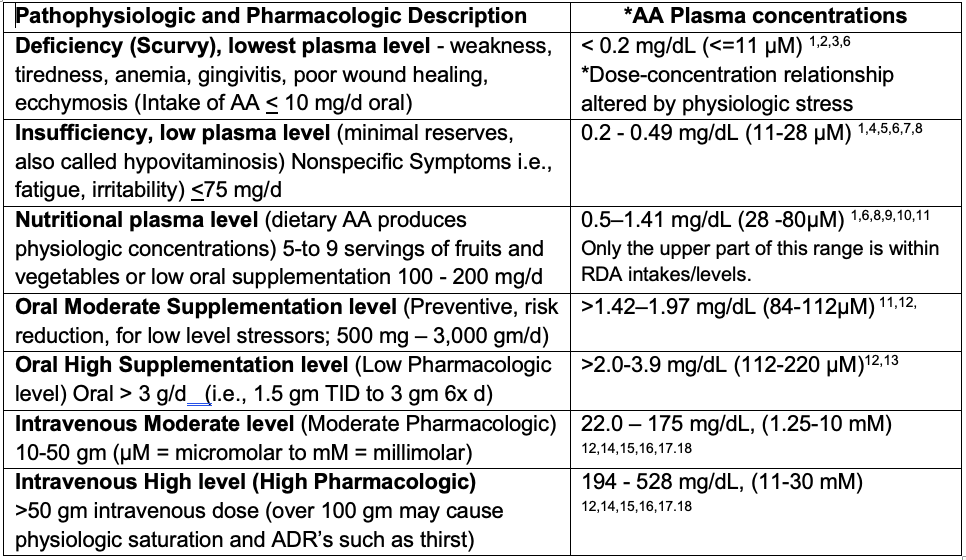
Table 2. Guide to AA Plasma Concentration Interpretation
Table References
- Lykkesfeldt et al. 2010,
- Food and Nutrition Board, 2000.
- Hagel et al. 2018
- Nyyssönen 1997.
- Smith 1987.
- Jacob 2002.
- Carr 2016.
- Krajcovicova-Kudlackova et al. 2007.
- Brubacher 2000.
- EFSA, NDA Panel. 2013.
- Levine et al. 1996.
- Padayatty et al. 2004.
- Cathcart 1981.
- Verrax J 2009.
- Riordan et al. 2004
- Tahahashi et al. 2012
- Ma 2014.
- Raymond 2016
Achieving Oral High Supplementation Levels
AA is available in liposomal formulation for oral consumption. Liposomes are a pharmaceutical delivery system consisting of microscopic sphere-shaped vesicles composed of phospholipid bilayers that encapsulate the active ingredient inside. The liposome can differ in particle size, composition, and charge, and drug carrier loaded with a variety of molecules and it is used for the purpose of protecting a compound from gastrointestinal degradation, reducing gastrointestinal adverse effects of the drug, and/or enhancing its absorption into the systemic circulation.
A bioavailability study conducted in the USA indicated that oral delivery of 4 g of AA encapsulated in liposomes produces circulating concentrations of AA that are 35% greater (AUC 0-4 h) than unencapsulated oral supplements and provides a similar level of protection from ischemia-reperfusion-mediated oxidative stress compared to unencapsulated oral and intravenous administrations (Davis et al., 2016). A different liposome increased half-life by 50% and elevated AUC 80%, and further evaluation of MTT tests in MCF7 cancer cell cultures demonstrated potency on the cellular level (Łukawski et al., 2020). Another clinical study of liposomal AA was demonstrated to be 1.77 times more bioavailable than non-liposomal AA (Gopi &Balakrishnan, 2020).
In summary, oral liposomes provide an enhanced bioavailability while improving tolerance. Presumably, the tissue distribution should be different because it may not entirely depend on the same transport mechanisms (glut, SVCT). These pharmacokinetic differences in distribution may impact the duration of action and may provide some therapeutic benefits.
Conclusion
In addition to the remarkable track safety record of intravenous and oral AA, there are a range of favorable physiological and pharmacological actions of AA in managing a variety of conditions.
In conclusion, AA is an essential nutrient responsible for an immense variety of physiological processes. National surveys in the USA and other countries have reported that 40% of the population has inadequate ingestion of this nutrient to meet the body’s basic demands, which is exacerbated in physiologically stressful conditions Given these the body’s needs are increased during physiologic stress and a large proportion of the population is presumably experienced transient or permanent AA insufficiency.
A vast body of research literature has demonstrated the pharmacologic activity of AA (antimicrobial, sepsis, anticancer, and others) when given at high levels especially intravenously. This notion that nutrients at higher concentrations can have additional pleiotropic actions is the essence of orthomolecular medicine and can be referred to as orthomolecular pharmacology. The proposed guide for plasma AA concentration can help the clinician to interpret the current condition of the patient and serve as a clinical guide, especially when applying intravenous AA as an adjunctive therapy.
Dedication
To the high dose vitamin C pioneers that gave us the light of understanding: Drs. Frederick R. Klenner, Robert F. Cathcart, Linus C. Pauling, Abram Hoffer and Hugh D. Riordan. Also, to our youngest and bravest inspiration that gave us the needed focus to never miss a step and the necessary courage to keep fighting: Gladys Isabel Rodriguez
Acknowledgement
We are grateful to Dr. Steve Hickey for revising the manuscript and making insightful and informative remarks.
References
Aditi A and Graham DY (2012). Ascorbic Acid, gastritis, and gastric disease: a historical review and update. Digestive diseases and sciences, 57(10), 2504–2515.
Adrover-López PA, Gonzalez MJ, Miranda-Massari JR, Duconge J and Berdiel M (2016). Inflammatory Sequelae After Chikungunya Virus Infection: Proposed. J Restorative Med, 5:39-45.
Ali SM, Chakraborty SK (1989). Role of plasma ascorbate in diabetic microangiopathy. Bangladesh Med Res Counc Bull,15(2):47-59.
Ames BN (2004). A role for supplements in optimizing health: the metabolic tune-up. Arch Biochem Biophys.,423(1):227-34.
Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ (1980). The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr, 33, 71–76.
Anderson R (1982). Effects of ascorbate on normal and abnormal leucocyte functions. Int J Vitam Nutr Res Suppl., 23:23-34.
Amato G (1937). Azione dell’acido ascorbico sul virus fisso della rabia e sulla tossina tetanica. Giornale di Batteriologia, Virologia et Immunologia (Torino), 19:843-847.
Baur H (1952). Poliomyelitis therapy with ascorbic acid: Helv Med Acta., 19(4-5):470-4.
Ballaz SJ, Rebec GV. Neurobiology of vitamin C: Expanding the focus from antioxidant to endogenous neuromodulator. Pharmacol Res. 2019 Aug;146:104321. doi: 10.1016/j.phrs.2019.104321. Epub 2019 Jun 20. PMID: 31229562.
Blaszczak W, Barczak W, Masternak J, Kopczyński P, Zhitkovich A, Rubiś B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules. 2019 Jan 28;24(3):453. doi: 10.3390/molecules24030453. PMID: 30695991; PMCID: PMC6384696.
Brabson JP, Leesang T, Mohammad S, Cimmino L. Epigenetic Regulation of Genomic Stability by Vitamin C. Front Genet. 2021 May 4;12:675780. doi: 10.3389/fgene.2021.675780. PMID: 34017357; PMCID: PMC8129186.
Banhegyi G, Braun L, Csala M, Puskas F, Mandl J (1997). Ascorbate metabolism and its regulation in animals. Free Rad Biol Med, 23, 793–803.
Banic S (1975). Prevention of rabies by AA. Nature,13;258(5531):153-4.
Berger MM (2015). Oudemans-van Straaten HM. AA supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care, 18(2):193-201.
Beyer RE (1994). The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme QJ Bioenerg Biomembr, 26(4):349-58.
Bode AM, Cunningham L, Rose RC (1990). Spontaneous decay of oxidized ascorbic acid (dehydro-L-ascorbic acid) evaluated by high-pressure liquid chromatography. Clin Chem, 36, 1807–1809.
Boel A, Veszelyi K, Németh CE, Beyens A, Willaert A, Coucke P, Callewaert B, Margittai É. Arterial Tortuosity Syndrome: An Ascorbate Compartmentalization Disorder? Antioxid Redox Signal. 2021 Apr 10;34(11):875-889. doi: 10.1089/ars.2019.7843. Epub 2019 Nov 14. PMID: 31621376.
Bozonet SM, Carr AC, Pullar JM, Vissers MCM (2015). Enhanced human neutrophil AA status, chemotaxis and oxidant generation following dietary supplementation with AA-rich SunGold kiwifruit. Nutrients, 7, 2574–2588.
Brubacher D, Moser U, Jordan P (2000). AA concentrations in plasma as a function of intake: a meta-analysis. Int J Vitam Nutr Res, 70(5):226-37.
Cahill L, Corey PN and El-Sohemy A (2009). AA deficiency in a population of young Canadian adults. Am J Epidemiol, 170: 464–471.
Cai Y, Li YF, Tang LP, Tsoi B, Chen M, Chen H, Chen XM, Tan RR, Kurihara H, He RR (2015). A new mechanism of AA effects on A/FM/1/47(H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed Res Int, 2015:675149.
Campbell EJ, Vissers MCM, Wohlrab C, Hicks KO, Strother RM, Bozonet SM, Robinson BA, Dachs GU (2016). Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Rad Biol Med, 99():451-462.
Carr AC, Pullar JM, Bozonet SM, Vissers MC (2016). Marginal Ascorbate Status (Hypovitaminosis C) Results in an Attenuated Response to AA Supplementation. Nutrients, 3;8(6). pii: E341.
Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017 Nov 3;9(11):1211. doi: 10.3390/nu9111211. PMID: 29099763; PMCID: PMC5707683.
Carroll RA, Chung RY, Prasad DP, Grant RW, Mitmesser SH (2020). Inadequacy of Immune Health Nutrients: Intakes in US Adults, the 2005–2016 NHANES. Nutrients, 12(6): 1735.
Cathcart RF (1981). The Method of Determining Proper Doses of AA for the Treatment of Disease by Titrating to Bowel Tolerance Othomolecular Psychiatry,10(2)125-132.
Chen S, Zhao W, Zhang B, Jia Y, Wu S, Zhong B, Yu X, Wang X, Hao Y, ang H, Zhao Y, Mizuno K, Bu H, Tseng Y (2019). Clinical Effect of Intravenous AA on Viral Myocarditis in Children: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med, 23; 2019:3082437.
Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M (2005). Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A, 102(38):13604-9.
Cheng LL, Liu YY, Li B, Ran PX (2012). An in vitro study on the pharmacological ascorbate treatment of influenza virus. Zhonghua Jie He He Hu Xi Za Zhi, 35(7):520–23.
Cheng LL, Liu Y, Li B, Ye F, Ran P (2014). [Pharmacologic ascorbate treatment of influenza in vivo]. Zhonghua Jie He He Hu Xi Za Zhi,37(5):356-9.
Dalton WL (1962). Massive Doses of AA in the treatment of Viral Diseases: J Indiana State Med Assoc, 55:1151-4.
Davis JL, Paris HL, Beals JW, Binns SE, Giordano GR (2016) et al. Liposomal-encapsulated Ascorbic Acid: Influence on AA Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury Nutr Metab Insights, 20;9:25-30.
Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, Mercolini L, Remião F, Nováková L, Mladěnka P, On Behalf Of The Oemonom. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients. 2021 Feb 13;13(2):615. doi: 10.3390/nu13020615. PMID: 33668681; PMCID: PMC7918462.EFSA NDA Panel (2013). Scientific opinion on dietary reference values for AA. EFSA J, 11,3418.
Emadi N, Nemati MH, Ghorbani M, Allahyari E (2019). The Effect of High-Dose AA on Biochemical Markers of Myocardial Injury in Coronary Artery Bypass Surgery. Braz J Cardiovasc Surg, 34(5):517-524.
Eremin O, Marshall V (1977). Complications of intravenous therapy: reduction by buffering of intravenous fluid preparation. Med J Aust, 2: 528-531.
Feigen G, Smith B, Dix C, Flynn CJ, Peterson NS, Rosenberg LT, Pavlović S, Leibovitz B (1982). Enhancement of antibody production and protection against systemic anaphylaxis by large doses of AA. Res Comm Chem Pathol Pharmacol, 38:313-333.
Fowler AA 3rd et al., (2019) Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 322(13):1261-1270. doi: 10.1001/jama.2019.11825. Erratum in: JAMA. 2020 Jan 28;323(4):379. PMID: 31573637; PMCID: PMC6777268.
Fritz H, Flower G, Weeks L, Cooley K, Callachan M, McGowan J, Skidmore B, Kirchner L, Seely D. (2014). Intravenous AA and cancer. Integrative cancer therapies, 13(4), 280–300.
Fukushima R, Yamazaki E (2010). Ascorbic Acid requirement in surgical patients. Curr Opin Clin Nutr Metab Care, 13(6):669-76.
Fujita M, Hatori N, Shimizu M, Yoshizu H, Hatori N, Shimizu M, Yoshizu H, Segawa D, Kimura T, Iizuka Y, Tanaka S. (2000). Neutralization of prostaglandin E1 intravenous solution reduces infusion phlebitis. Angiology, 51:719-723.
Furuya A, Uozaki M, Yamasaki H, Arakawa T, Arita M, Koyama AH (2008). Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int J Mol Med, 22(4):541-5.
Gallie DR. L-ascorbic Acid: a multifunctional molecule supporting plant growth and development. Scientifica (Cairo). 2013;2013:795964. doi: 10.1155/2013/795964. Epub 2013 Jan 17. PMID: 24278786; PMCID: PMC3820358.
Gao YL, Lu B, Zhai JH, Liu YC, Qi HX, Yao Y, Chai YF, Shou ST (2017). The Parenteral AA Improves Sepsis and Sepsis-Induced Multiple Organ Dysfunction Syndrome via Preventing Cellular Immunosuppression. Mediators Inflamm, 2017:4024672.
Gazitua R, Wilson K, Bistrian BR, Blackburn GL (1979). Factors determining peripheral vein tolerance to amino acid infusions. Arch Surg, 114: 897-900
German Nutrition Society (DGE) (2015). New Reference Values for AA Intake. Ann Nutr Metab, 67(1):13-20.
Greer E (1955). AA in acute poliomyelitis Med Times, 83(11):1160-1.
González MJ, Mora EM, Miranda-Massari JR, Matta J, Riordan HD, Riordan NH (2002). Inhibition of human breast carcinoma cell proliferation by ascorbate and copper. P R Health Sci J, 21(1):21-3.
González MJ, Berdiel MJ, Miranda-Massari JR, Duconge J. Rodríguez-López JL, Adrover-López PA (2016). High Dose Intravenous AA Treatment for Zika Fever. J Orthomolec Med, 31(1).
Gonzalez MJ, Berdiel MJ, Duconge J, Levy TE, Alfaro IM, Morales-Borges R, Marcial-Vega V, Olalde J (2018). High Dose Intravenous AA and Influenza: A Case Report. J Orthomolec Med, 33(3):1-3.
Gonzalez MJ, Berdiel MJ, Olalde J (2020). Miranda-Massari JR, Marcial-Vega VA, Aponte A. Intravenous AA and an orthomolecular protocol as therapy for COVID19: A case report. J Orthomolec Med, 35(1).
González MJ, Rosario-Pérez G, Guzmán AM, Miranda-Massari JR, Duconge J, Lavergne J, Fernandez N, Ortiz N, Quintero A, Mikirova N, Riordan NH, Ricart CM. Mitochondria, Energy and Cancer: The Relationship with Ascorbic Acid. J Orthomol Med. 2010;25(1):29-38. PMID: 23565030; PMCID: PMC3615720.
Gopi S, Balakrishnan P (2020). Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (AA) and their enhanced bioavailability. J. Liposome Res, 6;1-9.
Gugliandolo A, Gangemi C, Calabrò C, Vecchio M, Di Mauro D, Renis M, Ientile R, Currò M, Caccamo D. Assessment of glutathione peroxidase-1 polymorphisms, oxidative stress and DNA damage in sensitivity-related illnesses. Life Sci. 2016 Jan 15;145:27-33. doi: 10.1016/j.lfs.2015.12.028. Epub 2015 Dec 11. PMID: 26685757.
Guo G, Chen Q, Luo G, Meng Z, Lei P, Chen P, Drisko JA. High Dose Intravenous Vitamin C as Adjunctive Therapy for COVID-19 Patients with Cancer: Two Cases. Life (Basel). 2022 Feb 24;12(3):335. doi: 10.3390/life12030335. PMID: 35330086; PMCID: PMC8953706.
Hagel AF, Albrecht H, Dauth W, Hagel W, Vitali F, Ganzleben I, Schultis HW, Konturek PC, Stein J, Neurath MF, Raithel M (2018). Plasma concentrations of ascorbic acid in a cross section of the German population. J Int Med Res, 46(1): 168–174.
Hickey DS, Orberts HJ and Cathcart RF (2005). Dynamic Flow: A New Model for Ascorbate. Journal of Orthomolecular Medicine, 20(4):237-244.
Hughes K, Ong CN (1998). Vitamins, selenium, iron, and coronary heart disease risk in Indians, Malays, and Chinese in Singapore. Journal of Epidemiology and Community Health, 52(3):181‐5.
Hoffer LJ, Robitaille L, Zakarian R, Melnychuk D, Kavan P, Agulnik J, Cohen V, Small D, Miller WH Jr. (2015). High-dose intravenous AA combined with cytotoxic chemotherapy in patients with advanced cancer: a phase I-II clinical trial. PLoS One 10:e0120228.
Holden M (1937). Further experiments on the inactivation of herpes virus by AA (l-ascorbic acid). J Immunol, 33:251-257.
Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020 Dec 7;12(12):3760. doi: 10.3390/nu12123760. PMID: 33297491; PMCID: PMC7762433.
Hudson EP, Collie JT, Fujii T, Luethi N, Udy AA, Doherty S, Eastwood G, Yanase F, Naorungroj T, Bitker L, Abdelhamid YA, Greaves RF, Deane AM, Bellomo R (2019). Pharmacokinetic data support 6-hourly dosing of intravenous AA to critically ill patients with septic shock. Crit Care Resusc, 21(4):236-42.
Jacob RA and Sotoudeh G (2002). AA function and status in chronic disease. Nutr Clin Care, 5:66-74.
Joffe MI, Sukha NR, Rabson AR (1983). Lymphocyte subsets in measles. Depressed helper/inducer subpopulation reversed by in vitro treatment with levamisole and ascorbic acid. J Clin Invest,72(3):971-80.
Jungeblut CW (1935). Inactivation of Poliomyelitis virus in vitro by crystalline AA (ascorbic acid): J Exp Med, 30; 62(4):517 -21.
Karam F (1953). Vitamins B1 and C in the treatment of mumps. Rev Bras Med,10(4):285-6.
Kishimoto Y, Saito N, Kurita K, Shimokado K, Maruyama N, Ishigami A (2013). Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochemical and biophysical research communications, 430(2), 579–584.
Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N, Kong JM, Hwang YI, Kang JS, Lee WJ (2013). AA Is an Essential Factor in the Anti-viral Immune Responses through the Production of Interferon-α/β at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw,13(2):70–74.
Kim SR, Ha YM, Kim YM, Park EJ, Kim JW, Park SW, Kim HJ, Chung HT, Chang KC (2015). Ascorbic acid reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and improves survival rate in septic mice by activation of Nrf2/HO-1 signals. Biochem Pharmacol, 95(4):279-89.
Kim GN, Yoo WS, Park MH, Chung JK, Han YS, Chung IY, Seo SW, Yoo JM, Kim SJ (2018). Clinical Features of Herpes Simplex Keratitis in a Korean Tertiary Referral Center: Efficacy of Oral Antiviral and Ascorbic Acid on Recurrence. Korean J Ophthalmol,32(5):353-360.
Klenner FR (1949). The Treatment of Poliomyelitis and Other Viral Diseases with AA, Southern Medicine, and Surgery, 111(7):209-14.
Klenner FR (1951). Massive Doses of AA and the Virus Diseases, J Southern Med and Surg, 113(4): 101-107.
Klenner FR (1971). Observations On the Dose and Administration of Ascorbic Acid When Employed Beyond the Range Of A Vitamin In Human Pathology. J Applied Nutr, 23(3&4):1-20.
Kligler IJ and Bernkopf H (1937) Inactivation of vaccinia virus by ascorbic acid and glutathione. Nature; 139:965-966.
Klenner F (1953). The use of AA as an antibiotic. J Applied Nutr, 6:274-278.
Klenner F (1971). Observations of the dose and administration of ascorbic acid when employed beyond the range of a vitamin in human pathology. J Applied Nutr, 23:61-88.
Klenner F (1974). Significance of high daily intake of ascorbic acid in preventive medicine. Journal of the International Academy of Preventive Medicine, 1:45-69.
Krajcovicova-Kudlackova M, Babinska K, Valachovicova M, Paukova V, Dusinska M, Blazicek P (2007). AA protective plasma value. Bratisl Lek Listy, 108(6):265-8.
Kuiper, C., & Vissers, M. C. (2014). Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression. Frontiers in oncology, 4, 359. https://doi.org/10.3389/fonc.2014.00359
Lee SJ, Jeong JH, Lee IH, Lee J, Jung JH, Park HY, Lee DH, Chae YS. Effect of High-dose Vitamin C Combined With Anti-cancer Treatment on Breast Cancer Cells. Anticancer Res. 2019 Feb;39(2):751-758. doi: 10.21873/anticanres.13172. PMID: 30711954.
Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR (1996). AA pharmacokinetics in healthy volunteers: evidence for a Recommended Dietary Allowance. Proc Natl Acad Sci U S A; 93:3704-9.
Levine M, Padayatty SJ, Espey MG (2011). AA: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr, 2(2):78-88.
Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R, Farbstein D, Pollak M, Soloveichik YZ, Strauss M, Alshiek J, Livshits A, Schwartz A, Awad H, Jad K, Goldenstein H. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010 Feb;12(2):293-304. doi: 10.1089/ars.2009.2793. PMID: 19659435.
Li W, Maeda N, Beck MA (2006). AA deficiency increases the lung pathology of influenza virus-infected gulo-/- mice. J Nutr, 136(10):2611-6.
Liu X, Khan A, Li H, Wang S, Chen X, Huang H (2021). Ascorbic acid in epigenetic reprogramming. Curr Stem Cell Res Ther. 10.2174/1574888X16666210714152730. Epub ahead of print. PMID: 34264189.
Lojkin M (1936). A study of ascorbic acid as an inactivating agent of tobacco mosaic virus. Contr. Boyce Thompson Inst. Pl. Res, 8: 455.
Lominski I (1936). Inactivation du bacteriophage par l’acide ascorbique. Compt. Rond. Soc. Biol., 122: 766.
Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghes L, Franks W, Lawson TC, Sauberlich HE (2003). Ascorbic acid dynamics in the seriously ill and injured. J Surg Res., 109(2):144-8. doi: 10.1016/s0022-4804(02)00083-5.
Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, Wellen KE, O’Rourke DM, Berger SL, Chan TA, Levine SL, Mellinghoff IK, Thompson CB (2012). IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature, 483: 474–478.
Luchtel RA, Bhagat T, Pradhan K, Jacobs WR Jr, Levine M, Verma A, Shenoy N. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc Natl Acad Sci U S A. 2020 Jan 21;117(3):1666-1677. doi: 10.1073/pnas.1908158117. Epub 2020 Jan 7. PMID: 31911474; PMCID: PMC6983418.
Łukawski M, Dałek P, Borowik T, Foryś A, Langner M, Witkiewicz W, Przybyło M (2020). New oral liposomal AA formulation: properties and bioavailability. J Liposome Res., 30(3):227-234.
Lykkesfeldt J (2006). Smoking depletes AA: Should smokers be recommended to take supplements? In: Halliwell B, Poulsen HE, editors. Cigarette Smoke and Oxidative Stress. Springer; Heidelberg, Berlin, pp. 237–260.
Lykkesfeldt J, Poulsen HE (2010). Is AA supplementation beneficial? Lessons learned from randomized controlled trials. Br J Nutr, 103, 1251–1259.
Lykkesfeldt J, Tveden-Nyborg P (2019). The Pharmacokinetics of AA. Nutrients , 11(10). pii: E2412.
Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, Amodio V, Ceruti T, Sassi F, Arena S, Abrignani S, D’Incalci M, Zucchetti M, Di Nicolantonio F, Bardelli A. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med. 2020 Feb 26;12(532):eaay8707. doi: 10.1126/scitranslmed.aay8707. PMID: 32102933.
Mahmoodpoor A, Shadvar K, Sanaie S, Hadipoor MR, Pourmoghaddam MA, Saghaleini SH. Effect of Vitamin C on mortality of critically ill patients with severe pneumonia in intensive care unit: a preliminary study. BMC Infect Dis. 2021 Jun 29;21(1):616. doi: 10.1186/s12879-021-06288-0. PMID: 34187382; PMCID: PMC8240083.
Mandl J, Szarka A, and Bánhegyi G (2009). AA: update on physiology and pharmacology. British Journal of Pharmacology,157:1097–1110.
Manivasagam T, Arunadevi S, Essa MM, SaravanaBabu C, Borah A, Thenmozhi AJ, Qoronfleh MW. Role of Oxidative Stress and Antioxidants in Autism. Adv Neurobiol. 2020;24:193-206. doi: 10.1007/978-3-030-30402-7_7. PMID: 32006361.
Marcial-Vega V, Gonzalez-Terron I, Levy TE (2017). Intravenous Ascorbic Acid and Hydrogen Peroxide in The Management Of Patients With Chikungunya. Bol Asoc Med,107(1):20-24.
Marik PE (2018). AA for the treatment of sepsis: the scientific rationale. Pharmacol Therapeut, 189:63–70.
Marik PE (2018) Hydrocortisone. Ascorbic acid and thiamine (HAT therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients; 10:1762.
Mastrangelo D, Pelosi E, Castelli G, Lo-Coco F, Testa U (2018). Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood Cells Mol Dis., 69:57-64.
May JM and Qu ZC (2005). Transport and intracellular accumulation of AA in endothelial cells: relevance to collagen synthesis. Archives of biochemistry and biophysics, 434(1): 178–186.
Mayland CR, Bennett MI, Keith Allan K (2005). AA deficiency in cancer patients. Palliat Med,19(1):17-20.
McGregor GP, Biesalski HK (2006). Rationale and impact of AA in clinical nutrition. Curr Opin Clin Nutr Metab Care.,9(6):697-703.
Michels AJ, Hagen TM, Frei B (2013). Review Human genetic variation influences AA homeostasis by altering AA transport and antioxidant enzyme function. Annu Rev Nutr, 33():45-70.
Mikirova N, Hunninghake R (2014). Effect of high dose AA on Epstein-Barr viral infection. Med Sci Monit, 20:725-32.
Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, Levine M (2012). Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One.,7(1):e29794.
Morgan AR, Cone RL, Elgert TM (1976). The mechanism of DNA strand breakage by AA and superoxide and the protective roles of catalase and superoxide dismutase. Nucleic Acids Res, 3(5):1139-49.
Murata A, Oyadomari R, Ohashi T, Kitagawa K (1975). Mechanism of inactivation of bacteriophage deltaA containing single-stranded DNA by ascorbic acid. J Nutr Sci Vitaminol (Tokyo),21(4):261-9.
Nakajima M, Kojiro M, Aso S, Matsui H, Fushimi K, Kaita Y, Goto H, Yamaguchi Y, Yasunaga H (2019). Effect of high-dose AA therapy on severe burn patients: a nationwide cohort study. Crit Care, 12;23(1):407.
Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q (2014). High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy.Sci Transl Med, 6(222):222ra18.
Mikirova N, Rogers A, Casciari J, Taylor P (2012). Effect of high dose intravenous ascorbic acid on the level of inflammation in patients with rheumatoid arthritis. Modern Res in Inflammation,1(02):26-32.
Mitsuzumi H, Kusamiya M, Kurimoto T, Yamamoto I (1998). Requirement of cytokines for augmentation of the antigen-specific antibody responses by ascorbate in cultured murine T-cell-depleted splenocytes. Jpn J Pharmacol,78(2):169-79.
Nerella NG, Block LH, Noonan PK (1993). The impact of lag time on the estimation of pharmacokinetic parameters. I. One-compartment open model. Pharm Res.,10(7):1031-6. doi: 10.1023/a:1018970924508. PMID: 8104333.
Nielsen TK, Højgaard M, Andersen JT, Poulsen HE, Lykkesfeldt J, Mikines KJ (2015). Elimination of ascorbic acid after high-dose infusion in prostate cancer patients: a pharmacokinetic evaluation. Basic Clin Pharmacol Toxicol,116(4):343-8.
Niki E (1987). Interaction of ascorbate and alpha tocopherol. Ann N Y Acad Sci., 498:186-99.
Nishikimi M, Yagi K (1991). Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am J Clin Nutr,54(6 Suppl):1203S-1208S.
Nyyssönen K, Parviainen MT, Salonen R, Tuomilehto J, Salonen JT (1997). AA deficiency and risk of myocardial infarction: prospective population study of men from eastern Finland. BMJ, 314:634–8.
Orens S (1983). Hepatitis B—A ten Day “Cure”. A personal History: Bull Phila Cty Dent Socc., 48(6):4-5.
Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M (2004). AA pharmacokinetics: implications for oral and intravenous use. Ann Intern Med, 140(7):533-7.
Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M (2010). AA: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One, 5(7): e11414.
Pandipati S, Driscoll JE, Franceschi RT. Glucocorticoid stimulation of Na+-dependent ascorbic acid transport in osteoblast-like cells. J Cell Physiol. 1998 Jul;176(1):85-91. doi: 10.1002/(SICI)1097-4652(199807)176:1<85::AID-JCP10>3.0.CO;2-N. PMID: 9618148.
Pintér Ö, Hardi P, Nagy T, Gasz B, Kovács V, Arató E, Sínay L, Lénárd L, Jancsó G. The role of GST polymorphism in reperfusion-induced oxidative stress, inflammatory responses, and clinical complications after surgical and percutaneous coronary intervention. Clin Hemorheol Microcirc. 2017;66(3):261-272. doi: 10.3233/CH-170270. PMID: 28550240.
Pozzer D, Favellato M, Bolis M, Invernizzi RW, Solagna F, Blaauw B, Zito E. Endoplasmic Reticulum Oxidative Stress Triggers Tgf-Beta-Dependent Muscle Dysfunction by Accelerating Ascorbic Acid Turnover. Sci Rep. 2017 Jan 20;7:40993. doi: 10.1038/srep40993. PMID: 28106121; PMCID: PMC5247721.
Pozzer D, Invernizzi RW, Blaauw B, Cantoni O, Zito E. Ascorbic Acid Route to the Endoplasmic Reticulum: Function and Role in Disease. Antioxid Redox Signal. 2021 Apr 10;34(11):845-855. doi: 10.1089/ars.2019.7912. Epub 2020 Jan 22. PMID: 31867990.
Pecoraro RE, Chen MS (1987). Ascorbic acid metabolism in diabetes mellitus. Ann N Y Acad Sci.,498:248-58.
Polidori MC, Mecocci P, Frei B (2001). Plasma AA Levels Are Decreased and Correlated with Brain Damage in Patients with Intracranial Hemorrhage or Head Trauma. Stroke,32 (4): 898-902.
Preston AM, Rodríguez C, Rivera CE (2006). Plasma ascorbate in a population of children: influence of age, gender, AA intake, BMI and smoke exposure. P R Health Sci J.,25(2):137-42.
Raymond YC, Glenda CS, Meng LK (2016). Effects of High Doses of AA on Cancer Patients in Singapore: Nine Cases. Integr Cancer Ther.,15(2):197-204.
Ried K, Travica N, Sali A. The acute effect of high-dose intravenous vitamin C and other nutrients on blood pressure: a cohort study. Blood Press Monit. 2016 Jun;21(3):160-7. doi: 10.1097/MBP.0000000000000178. PMID: 26910646; PMCID: PMC4864764.
Reider CA, Chung RY, Devarshi PP, Grant RW, Hazels Mitmesser S (2020). Inadequacy of Immune Health Nutrients: Intakes in US Adults, the 2005-2016 NHANES. Nutrients., 12(6):1735. doi: 10.3390/nu12061735. PMID: 32531972; PMCID: PMC7352522.
Richter HE, Loewen PC (1982). Rapid inactivation of bacteriophage T7 by ascorbic acid is repairable. Biochim Biophys Acta, 697(1):25-30.
Riordan HD, Riordan NH, Jackson JJ, Casciari JJ, Gonzalez MJ, Miranda-Massari JR, Mora EM, Rosario N and Rivera A (2004). Intravenous AA as a Chemotherapy Agent: A report on Clinical Cases. PR Health Sci J, 23:115-8.
Riordan HD, Hunninghake RB, Riordan NH, Jackson JJ, Meng X, Taylor P, Casciari JJ, González MJ, Miranda-Massari JR, Mora EM, Rosario N, Rivera A (2003). Intravenous ascorbic acid: protocol for its application and use. P R Health Sci J., 22(3):287-90. PMID: 14619456.
Riordan Clinic. https://riordanclinic.org/research-study/vitamin-c-research-ivc-protocol/ Accessed in November 29, 2021.
Robitaille L, Mamer OA, Miller WH Jr, Levine M, Assouline S, Melnychuk D, Rousseau C, Hoffer LJ (2009). Oxalic acid excretion after intravenous ascorbic acid administration. Metabolism., 58(2):263-9. doi: 10.1016/j.metabol.2008.09.023. PMID: 19154961; PMCID: PMC3482487.
Salo RJ, Cliver DO (1978). Inactivation of enteroviruses by ascorbic acid and sodium bisulfite. Appl Environ Microbiol, 36(1):68-75.
Savini I, Rossi A, Pierro C, Avigliano L, Catani MV (2008). SVCT1 and SVCT2: key proteins for AA uptake Amino Acids, 34(3):347-55.
Schencking M, Vollbracht C, Weiss G, Lebert J, Biller A, Goyvaerts B, Kraft K (2012). Intravenous AA in the treatment of shingles: results of a multicenter prospective cohort study. Med Sci Monit., 18(4):CR215-24.
Schleicher RL, Carroll MD, Ford ES, Lacher DA 2009. Serum AA and the prevalence of AA deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr, 90: 1252–1263.
Schwager J, Bompard A, Weber P, Raederstorff D (2015). Ascorbic acid modulates cell migration in differentiated HL-60 cells and peripheral blood leukocytes. Mol Nutr Food Res, 59(8):1513-23.
Sharma P, Raghavan SA, Saini R, Dikshit M (2004). Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: Modulatory effect of nitric oxide. J Leukoc Biol, 75: 1070–1078.
Shatzer AN, Espey MG, Chavez M, Tu H, Levine M, Cohen JI (2013). Ascorbic acid kills Epstein-Barr virus-positive Burkitt lymphoma cells and Epstein-Barr virus-transformed B-cells in vitro, but not in vivo. Leuk Lymphoma, 54(5):1069-78.
Sinclair AJ, Girling AJ, Gray I, Guen CLe, Lunec J, Barnett AH (1991). Diabetologia. Disturbed handling of ascorbic acid in diabetic patients with and without microangiopathy during high dose ascorbate supplementation, 34:171-175
Sinclair AJ, Taylor PB, Lunec J, Girling AJ, Barnett AH (1994). Low plasma ascorbate levels in patients with type 2 diabetes mellitus consuming adequate dietary AA. Diabet Med.,11(9):893-8.
Shenoy N, Bhagat T, Nieves E, Stenson M, Lawson J, Choudhary GS, Habermann T, Nowakowski G, Singh R, Wu X, Verma A, Witzig TE (2017). Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J., 7, e587.
Shilotri PG (1977). Phagocytosis and leukocyte enzymes in ascorbic acid deficient guinea pigs. J Nutr, 107, 1513–1516.
Singh-Mallah G, Nair S, Sandberg M, Mallard C, Hagberg H. The Role of Mitochondrial and Endoplasmic Reticulum Reactive Oxygen Species Production in Models of Perinatal Brain Injury. Antioxid Redox Signal. 2019 Sep 20;31(9):643-663. doi: 10.1089/ars.2019.7779. Epub 2019 May 15. PMID: 30957515; PMCID: PMC6657303.
Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relationship with oxidative stress. Clin Lab. 2013;59(9-10):945-52. PMID: 24273915. ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59(9-10):945-52. PMID: 24273915.
Smith JL, Hodges RE (1987). Serum levels of AA in relation to dietary and supplemental intake of AA in smokers and nonsmokers. Ann N Y Acad Sci, 498, 144–152.
Stamford, NPJ (2012). Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. Journal of cosmetic dermatology, 11(4), 310–317.
Steers GJ, Carroll RS, O’Leary BR, Cullen JJ (2021). Epigenetic effects of pharmacologic ascorbate. Oncotarget., 12(9): 876–877.
Stephenson CM, Levin RD, Spector , Lis GG (2013). Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol., 72, 139–146.
Stone I (1980). The possible role of mega-ascorbate in the endogenous synthesis of interferon. Med Hypotheses, 6(3):309-14.
Sobala GM, Schorah CJ, Sanderson M, Dixon MF, Tompkins DS, Godwin P, Axon AT. (1989). Ascorbic Acid in the Human Stomach. Gastroenterology. 97:357-83
Sorice A, Guerriero E, Capone F, Colonna G, Castello G, Costantini S (2014). Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev Med Che, 14(5):444-452.
Sun L, Igarashi T, Tetsuka R, Li YS, Kawasaki Y, Kawai K, Hirakawa H, Tsuboi K, Nakamura AJ, Moritake T (2019). Pilot clinical study of ascorbic acid treatment in cardiac catheterization. J Radiat Res, 60(5):573-578.
Takahashi H, Mizuno H, Yanagisawa A (2012). High-dose intravenous AA improves quality of life in cancer patients. Personal Med Universe, 1:49–53.
Tanzer F, Ozalp I (1993). Leucocyte ascorbic acid concentration and plasma ascorbic acid levels in children with various infections. Mater Med Pol., 25(1):5-8.
Tomasa-Irriguible TM, Bielsa-Berrocal L (2021). COVID-19: Up to 82% critically ill patients had low AA values. Nutr J, 20, 66.
Turner G (1964). Inactivation of vaccinia virus by ascorbic acid. J Gen Microbiol, 35:75-80.
Vilchèze C, Kim J, Jacobs WR Jr (2018). AA Potentiates the Killing of Mycobacterium tuberculosis by the First-Line Tuberculosis Drugs Isoniazid and Rifampin in Mice. Antimicrob Agents Chemother, 62(3). pii: e02165-17.
Verrax J, Calderon PB (2009). Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Rad Biol & Med, 47;32-40.
Vissers MCM, Das AB (2018). Potential Mechanisms of Action for AA in Cancer: Reviewing the Evidence.Front Physiol., 9:809.
Villalpando S, Montalvo‐Velarde I, Zambrano N, García‐Guerra A, Ramírez‐Silva CI, Shamah‐Levy T, Rivera JA (2003). Vitamins A, and C and folate status in Mexican children under 12 years and women 12‐49 years: a probabilistic national survey. Salud Publica de Mexico, 45(Suppl 4): S508‐19.
Vollbracht C, Raithel M, Krick B, Kraft K, Hagel AF. Intravenous vitamin C in the treatment of allergies: an interim subgroup analysis of a long-term observational study. J Int Med Res. 2018 Sep;46(9):3640-3655. doi: 10.1177/0300060518777044. Epub 2018 Jun 27. PMID: 29950123; PMCID: PMC6136002.
Vorilhon P, Arpajou B, Vaillant Roussel H, Merlin É, Pereira B, Cabaillot A (2019). Efficacy of AA for the prevention and treatment of upper respiratory tract infection. A meta-analysis in children. Eur J Clin Pharmacol, 75(3):303-311.
Yoshikawa Y, Suzuki M, Chen N, Zinchenko AA, Murata S, Kanbe T, Nakai T, Oana H, Yoshikawa K. Ascorbic acid induces a marked conformational change in long duplex DNA. Eur J Biochem. 2003 Jul;270(14):3101-6. doi: 10.1046/j.1432-1033.2003.03699.x. PMID: 12846844.
Young JI, Züchner S, Wang G (2015). Regulation of the Epigenome by AA. Annu Rev Nutr., 35:545-64. doi: 10.1146/annurev-nutr-071714-034228. Epub 2015 May 6. PMID: 25974700; PMCID: PMC4506708.
Yun J, Johnson JL, Hanigan CL, Locasale JW (2012). Interactions between epigenetics and metabolism in cancers. Front Oncol, 2:163.
Zeneli L, Sekovanić A, Ajvazi M, Kurti L, Daci N (2016). Alterations in antioxidant defense system of workers chronically exposed to arsenic, cadmium and mercury from coal flying ash. Environ Geochem Health., 38(1):65-72.
Zureick M (1950). Therapy of herpes and herpes zoster with intravenous AA. J Prat Rev Gen Clin Ther, 64(48):586.


