Abbreviations
AD: Anxiety Disorders
ADHD: Attention Deficit Hyperactivity Disorder
ASD: Autism Spectrum Disorder
CAM: Complementary and Alternative Medicine
CMT: Chronic Motor Tics
CVT: Chronic Vocal Tics
FMT: Faecal microbiota transplantation
FODMAP: Fermentable Oligosaccharides, Disaccharides, Monosaccharides And Polyols
LD: Language Disorders
OCD: Obsessive Compulsive Disorder
QOL: Quality of Life
SIBO: Small Intestinal Bacterial Overgrowth
SPD: Sensory Processing Disorder
TD-NOS: Tic Disorder Not Otherwise Specified
TS: Tourette Syndrome
YGTSS: Yale Global Tic Severity Scale
Introduction
Definition
A tic is a “sudden, repetitive, nonrhythmic, stereotyped motor movement or vocalization involving discrete muscle groups” that can present with different intensity, frequency, and duration (APA, 1980). Tics can occur as simple (an eye blink) or complex (a body contortion) behaviours (Singer, 2010). Vocal tics can present as sniffing, throat clearing, inappropriate use of language (coprolalia) or repeating another person’s speech (echolalia).
Disorders and Comorbidities
Tics are most often associated with Tourette syndrome (TS), though “pure” TS only occurs in 10% of cases (Karagiannidis et al., 2016). Apart from TS, tics can be part of many other diagnoses: transient tic disorder (TTD) (tics that are present for less than a year), chronic motor tics (CMT), chronic vocal tics (CVT) (isolated motor or vocal tics that are present for more than a year), tic disorders not otherwise specified (TD-NOS) (tics with an adult onset or tics that are connected to drug use/withdrawal) (Singer, 2010). In addition, tics are highly comorbid to a multitude of psychopathologies: obsessive compulsive disorder (OCD) – up to 89% of patients; attention deficit hyperactivity disorder (ADHD) – up to 50%, behavioural, anxiety and depression disorders – up to 76% (Bloch & Leckman, 2009; Khalifa & Von Knorring, 2006; Singer, 2010; Worbe et al., 2010). In a sample of 105 patients diagnosed with autism spectrum disorder (ASD), 11% displayed TS and another 11% CMT (Canitano & Vivanti, 2007).
Prevalence and Clinical Course
Tics have been reported in up to 20% of children (Singer, 2010). Initially described as a rare condition TS is becoming increasingly common: studies suggest a prevalence between 1 and 4% (Mohammadi et al., 2021; Robertson, 2008; Scahill, Sukhodolsky, Williams, & Leckman, 2005). Contrary to what George Gilles de la Tourette initially described, tics are not stable across the lifespan: typically, they emerge around six years of age, worsen progressively until ten, then regress during adolescence (Leckman et al., 1998). However, recent studies suggest the highest prevalence of TS is among teenagers between 15 and 18 years of age (Mohammadi et al., 2021). Bloch and Leckman (2009) report that over a third of adults have mild tics, and Singer (2010) points out: “about half of adults, reporting the resolution of tics, actually had tics confirmed on direct observation” (p.540). Furthermore, while most studies concur with the post-adolescence regression perspective, Schaefer, Chow, Louis and Robakis (2017) report cases of tic re-emergence in adulthood, after a latent period.
Quality of Life
Tics impact quality of life (QOL). Studies show significant impacts on academic and professional performance, as well as emotional and social status (Eddy, Rickards & Cavanna, 2010; Evans, Seri & Cavanna, 2016). Up to 70% of patients with TS do not reach expected educational outcomes (Singer, 2010), and self-injurious behaviours occur in at least 30% of cases (Robertson, Trimble & Lees, 1989). In addition, most authors suggest that comorbid disorders influence QOL just as much, if not more, than the tics themselves (Bernard et al., 2009; Pringsheim, Lang, Kurlan, Pearce & Sandor, 2009). While the tics may become less frequent, comorbid symptoms are more likely to persist (Bloch et al., 2006).
Proposed Aetiologies
TS’s aetiology appears to be complex and multidimensional. Twin studies have provided strong evidence for the genetic basis of TS (Singer, 2010, Wu et al., 2021). The anatomy and pathophysiology of tics are thought to stem from cortico-basal ganglia–thalamo–cortical network dysfunction (Berardelli, Currà, Fabbrini, Gilio & Manfredi, 2003; Hashemiyoon, Kuhn & Visser-Vandewalle, 2016; Stern et al., 2000), and neurotransmitter disturbances have been highlighted (Wu et al., 2021). In addition, environmental (perinatal adverse events) and immunological (reactions to infections) aetiologies have been suggested (Robertson et al., 2017). Karagiannidis et al. (2016) state that this aetiological background is shared with the other neurodevelopmental disorders that TS patients tend to display: OCD, ADHD, ASD, LD, etc.
Treatments
Existing treatments are documented in Singer’s work (2010, pp. 543-556): behavioural approaches, surgical interventions, deep brain stimulation and a plethora of pharmaceutical drugs. The medications all have adverse effects, and none of them cure the patient (Hashemiyoon, Kuhn & Visser-Vandewalle, 2016). Combined with the significant prevalence of comorbidities and ineffectiveness of mainstream pharmacological treatments, it appears to be nearly impossible to relieve patients from all discomfort.
Diet, Nutrition, Gut Health and Immunity in TS
People with TS are fussy eaters, relying mainly on refined sugar and processed carbohydrates (Liang et al., 2015; Smith, Roger, Blisset, & Ludlow, 2019). Their urine samples contain high levels of excitatory substances, sugar, food dyes and flavorings (Zou et al., 2011). They suffer from nutritional deficiencies (Liang et al., 2015; Zimmer et al., 2012). Deficiencies in magnesium and vitamins B2 and B6 have been explored as possible causes for tics and neuromuscular hyperexcitability (Garcia-Lopez et al., 2009; Grimaldi, 2002; Planells et al., 1997). Low iron levels may be involved in the pathophysiology of tics, because iron has a significant impact on dopamine metabolism (Cortese et al., 2008). Dopaminergic alterations are believed to be a causal factor in Tourette’s (Hienert, Gryglewski, Stamenkovic, Kasper & Lanzenberger, 2018). Anaemia may be a causative factor in caudate putamen hypoplasia, which is known to increase both tic development and severity (Gorman, Zhu, Anderson, Davies & Peterson, 2006).
Excessive consumption and cravings for sugars and carbohydrates are indicative of gut dysbiosis (Campbell-McBride, 2020; Kanhere et al., 2021). In a recent study, Xi et al. (2021) have confirmed that “gut microbiota in children with TD is distinguishable from that of healthy individuals” (p.6). In particular, high levels of Bacteroides Plebeius and Ruminococcus Lactaris were found. These bacteria have been implicated in autoimmune conditions (Zhang et al., 2015), inflammatory diseases (Mondot et al., 2016) and autism (Dan et al., 2020). This concurs with findings by Quagliarello et al. (2018), who led a gut microbiota profiling in patients diagnosed with PANDAS and found clear signs of dysbiosis. Since the mother’s body is the source of the child’s microbiome (Mueller et al., 2015), maternal health provides further evidence: mothers of children with TS, CMT, CVT, ADHD, OCD and ASD often suffer from autoimmune diseases and inflammatory states (Han, Patel, Jones & Dale, 2021; Jones et al., 2021).
Research is establishing close connection between neurodevelopmental disorders and gut health and nutrition. For instance, in ADHD:
“the increase in dopamine (caused by sugar intake) might lead to a reduction in the number of D2 receptors and/or a decrease in extracellular dopamine itself. This could potentially lead to desensitization of the dopamine-signalling axis, meaning it is possible that children with ADHD may then ingest more sugar in an effort to correct the dopamine-deficient state. Therefore, children with TS, a population also identified as having dysfunction of the dopamine system, may also show a tendency to consume high sugar foods, which may in turn exacerbate tics” (Johnson et al., 2011, as cited in Ludlow & Rogers, 2017, p.8).
Studies have suggested an increase in tics following consumption of caffeine, refined sugars and alcohol (Davis & Osorio, 1998; Müller, Buddensiek, Geomelas, & Emrich, 2008; Schaefer et al., 2017).
Nutritional and CAM Interventions
Faecal microbiota transplantation (FMT) is showing promising results in tics: Zhao et al. (2020) observed five participants. Eight weeks after FMT, four had a reduction in tics by over 25% on the YGTSS (Yale Global Tic Severity Scale). The restoration of Bacteroides coprocola was particularly correlated with tics improvement. A British review article by Smith and Ludlow (2021) highlighted the increase in CAM (Complementary and Alternative Medicine) interventions in children with TS. Improvement was noted in 75% of supplement users (probiotics, omega-3, vitamins) for both tics and AD related symptoms. In their sample, 53% of parents who implemented sugar-free, dairy-free or gluten-free diets for their children observed at least one positive change. Similar results were found by Rodrigo et al. (2018) after one year on a gluten-free diet. Statistically significant (p = 0.001) reduction in measures of both tics (according to the YGTSS) and OCD (according to the Yale-Brown Obsessive Compulsive Scale) were observed. QOL of both adults and children improved significantly. Rodrigo, Huerta and Salas-Puig (2015) reported a case of a 13-year-old who, after ten years of tics and OCD, was completely symptom-free following a 2 months-long gluten-free diet. Symptoms had already dramatically decreased after one week on the diet. Garcia-Lopez et al. (2009) suggest that supplementation of vitamins B6 and magnesium improve symptoms in tic-related disorders. Wu et al. (2021) led a randomized controlled trial, using a probiotic PS128 (Lactobacillus plantarum) as a treatment for TS. Contrary to Liao et al.’s (2019) report, there were no significant results for tic-reduction, however ADHD symptoms were greatly reduced, improving QOL.
The Current Case Study
Recent discoveries of human microbiome and its gut-brain connections are becoming well established. Hence, research on the root causes of tic disorders is increasingly focussing on gut microbiota. Our gut is a promising target for therapeutic intervention (Cryan et al., 2019) because, contrary to our genome, gut microbiota are flexible and responsive to environmental changes (Lloyd-Price et al., 2017). Change in diet is particularly powerful (Leeming, Johnson, Spector & Le Roy, 2019), although host factors can determine the degree to which diets have an impact (Lkhagva, Chung, Ahn & Hong, 2021).
Authors are calling for researching alternative treatments for tic-related disorders (Zhao et al., 2020). Changes in diet and nutrition are central (Briguglio, Dell’Osso & Porta, 2021; Liang et al., 2015) as evidence is growing in that direction. According to Martino, Johnson, and Leckman (2020), dietary interventions should focus on rebalancing the gut microbiome. Yet, TS is receiving very little attention in this respect, especially compared to its comorbid disorders such as ASD and ADHD (Ludlow & Rogers, 2017). Mainstream drug-based treatments cause serious adverse effects: AD, depression, motor dysfunction, type 2 diabetes and others (Ludlow & Rogers, 2017; Scahil et al., 2016). Furthermore, Aripiprazole and Risperidone, commonly prescribed for TS, have been found to damage gut microbiota (Bahr et al., 2015; Cussotto et al., 2018). CAM interventions are gaining popularity, which is indicative of a growing distrust towards mainstream approaches (Silva, Munoz, Daniel, Barickman & Friedhoff, 1996), and more than 80% of patients do not consult their general practitioners before implementing alternative treatments (Smith & Ludlow, 2021).
Gluten-free diets and dairy-free diets have been the most explored in relationship to TS and have been reported to have a potential significant impact on symptoms (Rodrigo et al., 2018; Smith and Ludlow, 2021). In this study we aim to further explore dietary treatments for tics and tic-related disorders, focussing on gut health. The GAPS Nutritional Protocol, which we cite in this article, is grain-free, making it truly gluten-free. In this protocol, nutritional deficiencies are addressed through nutrient-dense organic foods, such as organ meats and gelatinous meats, seafood, fresh eggs, healthy fats, and fermented vegetables and beverages. Probiotics, central to gut health (Naureen, Farooq, Zahoor & Gilani, 2022), are provided through fermented foods and, sometimes, supplements. The GAPS Diet is not dairy-free, though it is possible to follow it without dairy products if necessary. Only well-fermented organic dairy foods are used. Fresh, unprocessed raw milk or cream are fermented at home for 24 hours, making them truly lactose-free. Fermentation breaks down casein and other proteins in the milk, rendering them harmless. Special attention is given to the neutralization of antinutrients in legumes and other plant foods (Akande, Doma, Agu, & Adamu, 2010). For a detailed description of the GAPS Diet please see Delaunay-Vagliasindi, Seneff, and Campbell-Mcbride (2021) and GAPS books by Dr N Campbell-McBride.
Methodology
Participants and Recruitment
Inclusion criteria were: 1. past display of tics and 2. implementation of the GAPS Nutritional Protocol. Participants were recruited through the databases of experienced healthcare practitioners who use the GAPS Nutritional Protocol daily in their medical practices (Shantih Coro and Becky Plotner). Twenty candidates, who met the inclusion criteria, were contacted, out of which six accepted to take part in the study. Reasons for declining were the fact that participation was time-consuming or would cause recollection of painful experiences. For those, who accepted to be included in the study, consent forms were signed guaranteeing anonymity.
Data Collection and Analysis
We had access to five of the participants’ blood work and different lab analyses prior to and following the GAPS Nutritional Protocol. Due to the retrospective nature of the study, we had little control over which blood counts had been collected. Some interesting values to explore, such as zinc and magnesium deficiencies, were unfortunately not available to us, or had not been systematically collected prior to and post-intervention.
We conducted unstructured interviews with all participants or their parents. Interviews lasted one hour on average. Participants were encouraged to share their medical history freely. Questions were never too specific or close-ended, to avoid influencing the participants’ answers. Interviews were recorded and notes were taken by the interviewer. The notes were translated into English for the Italian participants. Collected data were analysed and summarised (see Table 1). Particular attention was given to similarities between different cases. When similar elements emerged across participants, they were further discussed as clusters (see Table 3).
RESULTS
Comorbidities
The most common comorbidities in this sample were digestive issues and speech and language disorders (see Table 1 and 2). Participant 2 was diagnosed with Small Intestinal Bacterial Overgrowth (SIBO). Participant 3 suffered from daily morning vomiting for over three months. Participant 5 had such intense colic and diarrhoea that food was almost immediately excreted and found in the stool. Four participants suffered from dysgraphia and dyslexia. Participant 1 could only write with a pencil and in capital letters. Participant 3’s dysgraphia was so bad that this person was unable to write at all. Around 19 months, participant 5 regressed to a completely non-verbal state.
Attention deficit disorders and body weight issues were the second most present comorbidities in our sample. Participant 2 would get so distracted that he was unable to play football; he would forget to look at the ball and let the opponents score. Participants 1 and 3 were overweight, despite eating a normal diet and making efforts to lose weight. Participant 5 was so underweight that he was diagnosed with failure to thrive and put on a tube.
Other problems mentioned by the participants were: behavioural issues, AD, ASD and SPD, bedwetting, seizures, hallucinations, sleep issues and excessive sweating.
QOL
Together with motor and verbal tics, above mentioned symptoms affected every participant’s QOL. Schooling was disrupted, social life was impacted, and tensions emerged in families. The father of participant 1 avoided coming back home, because he could not stand the sounds his son made. The parents of participant 3 split up due to disagreements on how to handle their daughter’s plummeting health situation. Participants 1, 2, 3, and 4 struggled in their social interactions with their peers; participant 3 was bullied by other children so badly that she had to change schools. Participant 6 took hours to read a short text, because she would start over again each time sentences did not add up to three-syllable-increments.
Improvements
All participants’ tics and comorbidities improved following the GAPS Nutritional Protocol. The children who suffered from severe dysgraphia were able to go back to school. Those who were doing poorly academically due to ADHD were back on track. The children who were overweight lost weight and the participant who was severely underweight finally reached the age-appropriate percentiles. Tics improved in every participant, especially during the GAPS Introduction Diet. These improvements were observed within the first month from starting the protocol. Participants 1, 3 and 6 are still healing. Participant 6 says “even though the tics are still present, they feel different, like they are in the background (…) and I am very able to control them.” Participants 2 and 4 are tic-free with a few exceptions. Participant 2’s tics re-emerge when he gets a cold, and participant 4’s after consuming refined sugar. Participant 5 is now absolutely tic-free and considered to be a typically developing child.
These results highlight how important it is to “think across diagnostic categories” (Karagiannidis et al. 2016, p.218). Since all these improvements occurred concurrently, the idea that tics and their comorbidities hold a common aetiology – gut health – is supported by our findings.
Inflammation and Autoimmunity
Inflammatory and autoimmune states were reported in virtually every participant (see Table 1 and 3), further demonstrating that these patients have a compromised immune system and microbiome. Participant 2 suffered from repeated severe otitis from a young age. At the age of seven he suffered from such severe headaches that he had to stay in bed for days. When he was eight years old, he was hospitalised for pancreatitis. Participant 3 was diagnosed immunodeficient. Participant 5 was so chronically congested that he was unable to breastfeed.
Antibiotics and Medication
All participants received repeated antibiotic courses throughout their lives, except participant 6 (see Table 3). Being the only adult in the sample, less attention was given to tics and ADHD while she was growing up. Her parents never got her diagnosed, which spared her from medications. As an adult, she was suggested to take antibiotics multiple times, but she purposely avoided them. For participants 2 and 3, who were diagnosed with TS and PANS, the daily shots of penicillin were recommended for over two years. At one point, participant 3 was taking three different kinds of antibiotics daily. The antibiotics seemed to alleviate the tics for a while but did not help the crippling anxiety that comes with PANS. Antibiotics are well-known to cause damage to gut flora and gut health. So, it is not surprising that, after an initial improvement (which lasted a few weeks at best), the situation typically worsened and symptoms returned.
Distrust Towards Doctors
Broken relationship with medical professions was omnipresent in the participants and their families (see Table 3). All participants had terrible experiences and were treated disrespectfully on many occasions. Mothers were told that their own anxiety was causing their children’s illness. The patients were ignored, misdiagnosed, deprecated and discouraged. Drug prescriptions, antibiotics especially, were the main recommendation, despite participants reporting no improvement and wanting to dig deeper. It is disturbing to observe such a compromised relationship between the medical profession and patients.
Maternal Influence
Mothers of most participants in this study had chronic inflammatory disorders, cases 5 and 6 being the worst. Participant 5’s mother underwent domestic abuse while pregnant. Such acute stress is known to have direct impact on gut health and microbiome composition (Spadoni, Fornasa & Rescigno, 2017). Participant 6 was born vaginally and breastfed while her mother had a chronic yeast infection; she has suffered from chronic pain from a young age, and her older brother is diagnosed with ASD and multiple sclerosis. All mothers reported illnesses that improved in parallel with recovery of their children, as they implemented the diet for themselves too.
Nutritional Deficiencies
Vit D, Vit B9, creatinine, ferritin, lymphocytes, neutrophils, and cholesterol levels were abnormally high or low in some of our participants. After the GAPS Nutritional Protocol, six out of eight nutritional deficiencies were removed. Cholesterol levels and white blood cells counts returned to normal ranges (see Table 1, 4, 5, 6).
Diet Adherence
Going through the GAPS Introduction Diet is recommended before following the Full GAPS Diet. Participant 2, who had SIBO, did not follow this recommendation and started from the Full GAPS Diet (see Table 1); he also did not remove all starches, which is a strict requirement in the GAPS Protocol. Yet, his improvement is no less remarkable. He went from hallucination episodes and being unable to understand what his teacher would say to being successful at school. His enlarged lymph nodes shrank down to normal naturally without any medication.
But the most impressive results (complete recovery) were observed in participants 4 and 5. Participant 5 was two years old when his mother implemented the GAPS Nutritional Protocol. He is now considered healthy by his paediatrician and speech therapist. By the time he got tested for ASD (at the age of four years), all his ASD and SPD symptoms had disappeared, and his profile was declared one of a typically developing child.
The mother of participant 4 followed the GAPS Nutritional Protocol perfectly (the child was 7 years old at the time). She sourced the cleanest foods (sometimes driving three hours to get pasture-raised meat) and was meticulous in following the GAPS Diet strictly.
These cases highlighted the importance of intervening as soon as possible: the younger the child is when the GAPS Nutritional Protocol is implemented, the better the results. Adhering to the GAPS Diet as strictly as possible is also important. Parents of both participants 4 and 5 implemented the diet for the recommended two years and followed it rigorously.
The GAPS Diet should be followed for two years minimum, in some cases longer. All participants in our study reported re-emergence of tics when not-allowed foods were reintroduced too soon.
Obstacles to Diet Adherence
Our interview data suggests that GAPS Nutritional Protocol implementation and compliance are associated with family support. It is vital to have a supportive family that understands the reasons of the dietary change and does not hinder the process. The mother of participant 3 had marital problems due to her daughter’s illness, because the effort required was too much for her husband. Couples and families who were united in their effort to implement the GAPS Nutritional Protocol got the best results. Communication is also central. The mother of participant 4, who adhered to the diet very strictly, told us that she communicated with her son explaining thoroughly why they were making this change. She felt that this communication was vitally important; the child was so understanding and grateful that he never tried to ‘cheat’. This supports Singer’s (2010) conclusion: “factors that appear to correlate with positive outcomes, regardless of tic severity, include intelligence, coping and social skills, meaningful daily activities, and good family and social support” (p. 540).
Toxicity
Four participants mentioned the deleterious impact that a toxic environment can have on healing. Too much time spent in front of screens, including video games, scented candles, polluted tap water, pesticides (see Table 3) and other modern elements have slowed down their healing. This aspect is addressed in the GAPS Nutritional Protocol. Indeed, the GAPS Diet is only one aspect of the protocol; reducing toxicity in the environment is another central one (see Campbell-McBride, 2020). When the participants understood the impact of such factors, they removed them and observed multiple improvements.
Strengths and Limitations
Our study has limitations due to its retrospective nature. However, retrospective case studies are valuable in providing first evidence towards a hypothesis (Talari & Goyal, 2020). In addition, we counteracted biases by looking at quantitative data (blood counts) and spontaneously mentioned qualitative data. No precise close-ended questions were asked during interviews to avoid prompting. We believe that this study gives a good basis for further research, focussing on gut health in patients with tics and related disorders.
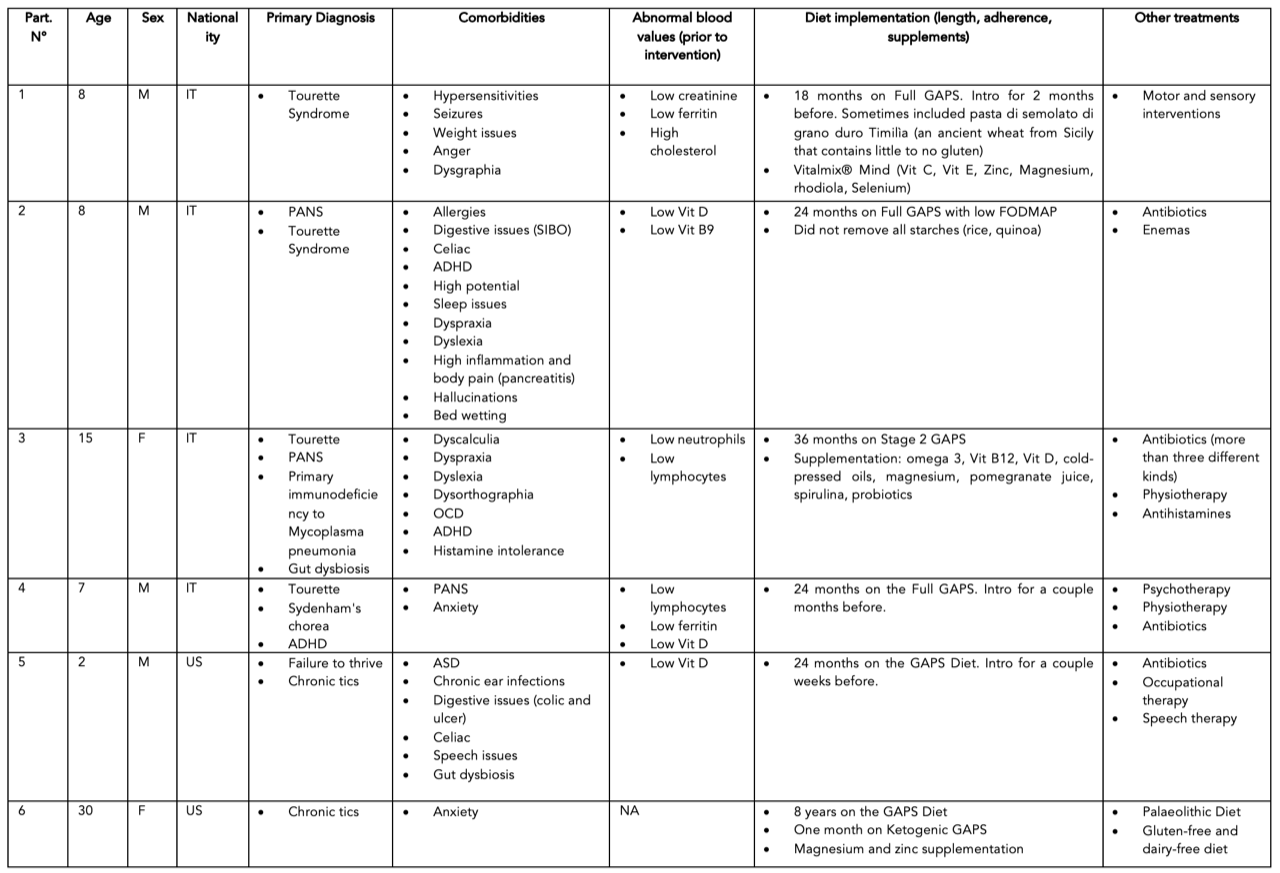
Table 1: Participants’ age, sex, nationality, diagnoses, comorbidities, and treatments.
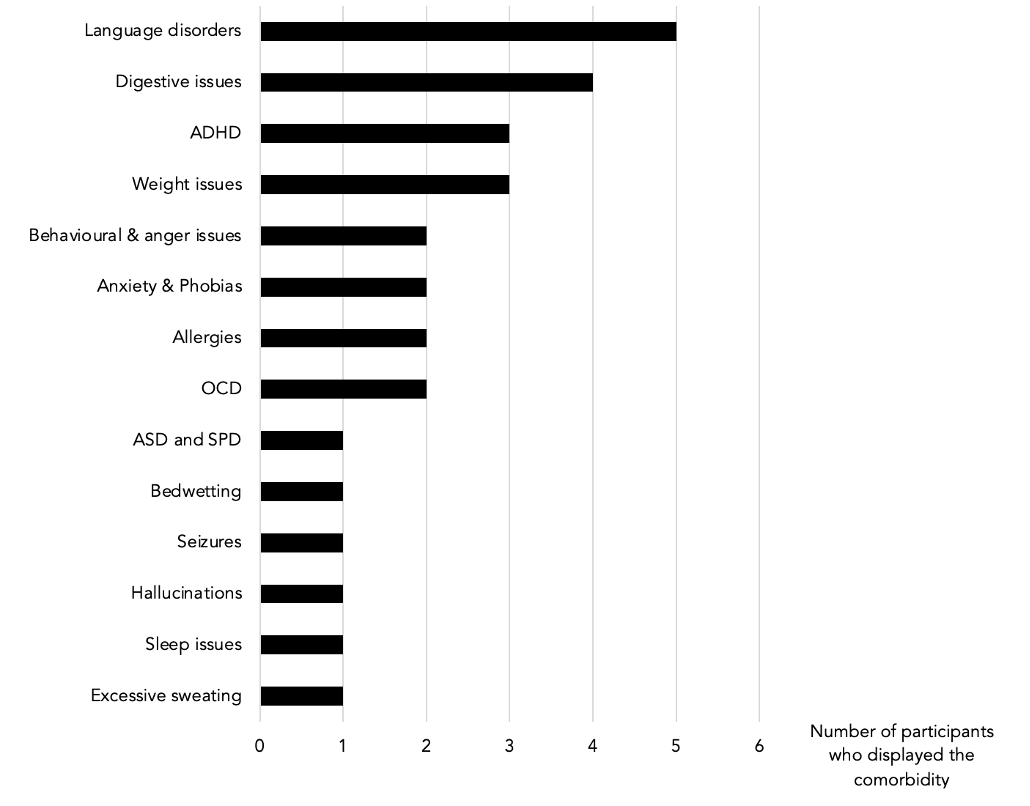
Table 2: Comorbidities spontaneously mentioned by participants.
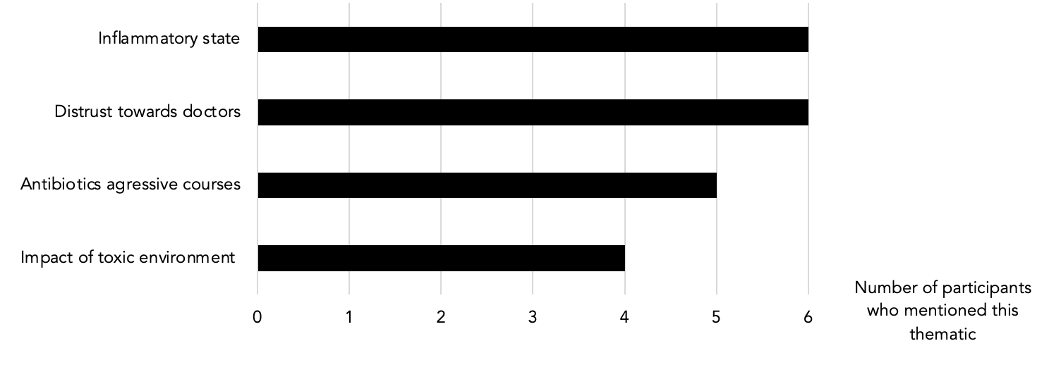
Table 3: Clusters (repeated non-prompted thematics during the interviews)
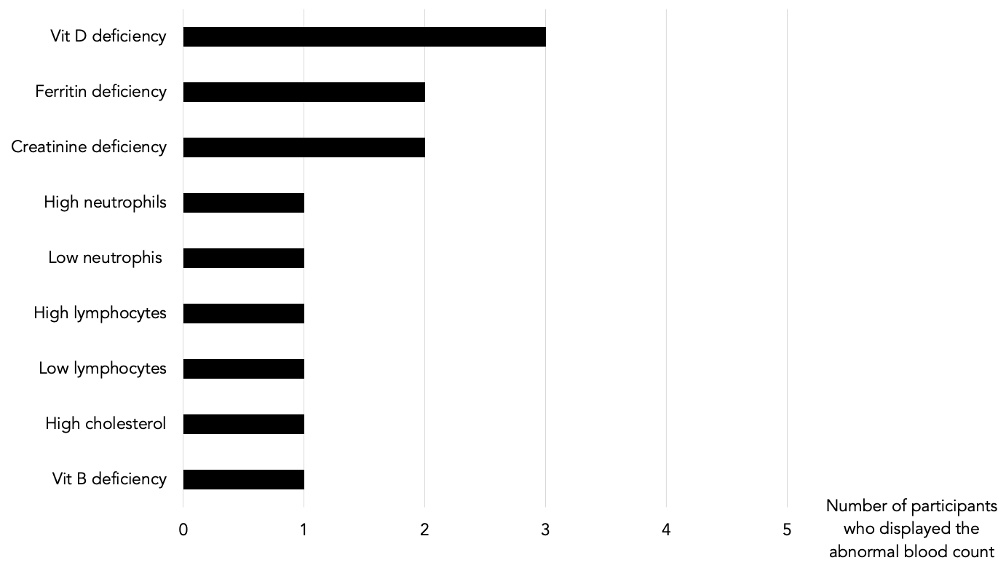
Table 4: Abnormal blood counts prior to GAPS intervention
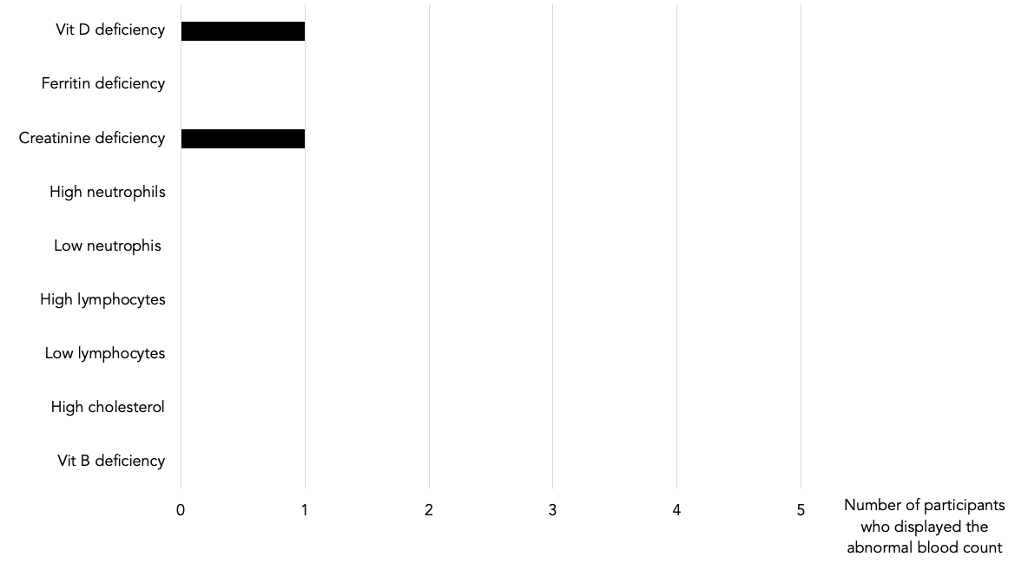
Table 5: Abnormal blood counts post GAPS intervention.
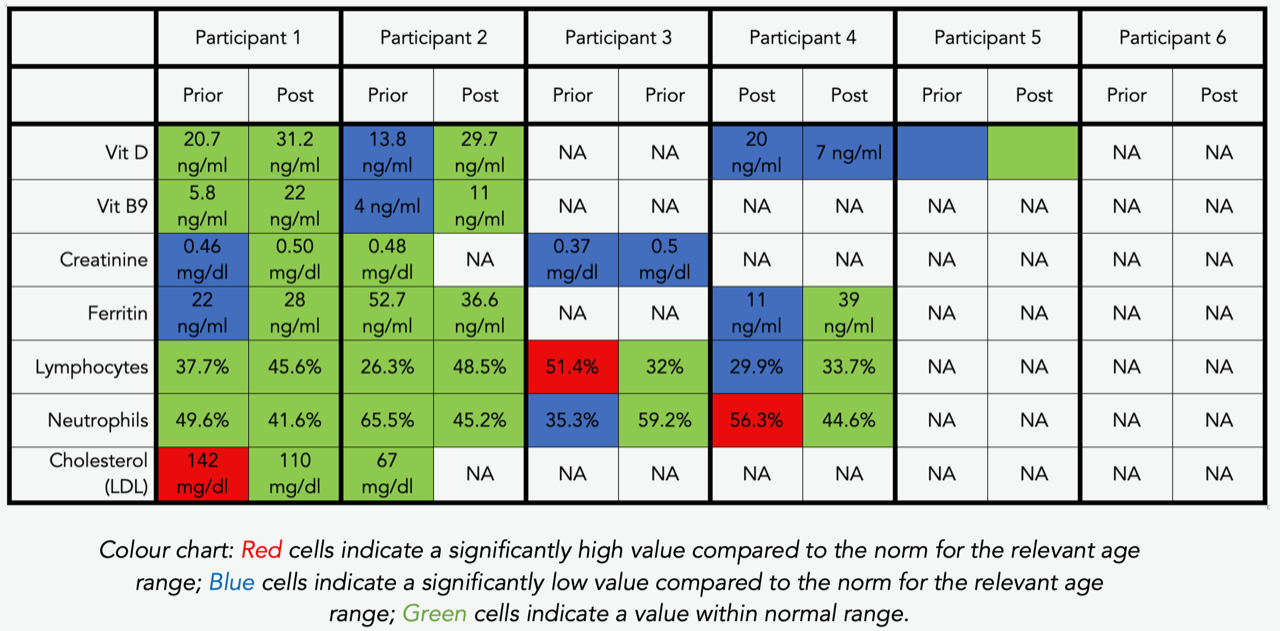
Table 6: Exact blood counts prior to and post intervention, per participant
Conclusion
Our data shows that the GAPS Nutritional Protocol, with its focus on gut health, provides a very promising treatment for tics and their comorbidities. Three participants are tic-free, while the other three had dramatic improvements and are still healing. All participants’ quality of life improved. Nutritional deficiencies were removed and blood counts normalised. Strict compliance with the GAPS Nutritional Protocol and early intervention led to best results. However, healing can be achieved even if this protocol is followed partially (removing sugar, grains and processed foods, while incorporating fermented foods). We advise against the use of antibiotic treatments for tic-related disorders, as they only alleviate some symptoms short-term, but worsen the long-term outcome and overall health of the patient. Our data supports previous findings, linking tic-related disorders with gut health, chronic inflammation and autoimmunity. We confirm the influence of mothers’ health during pregnancy. It is clear that both diet adherence and positive outcomes depend strongly on family support: coaching and psychological help should be offered to the patient and family members. Governments should consider funding dietary and CAM interventions for patients with tic-related disorders and their comorbidities.
Declarations
Consent for Publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patients. Copies of consent forms are available for review by the Editor of this journal.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Dr Natasha Campbell-McBride is the creator of the GAPS concept and the GAPS Nutritional Protocol.
Funding
No financial support has been received for the work reported.
Authors’ Contributions
Shantih Coro and Becky Plotner collected the data. Sophie Delaunay-Vagliasindi collected additional qualitative data, analyzed all data, and wrote the manuscript. Natasha Campbell-McBride and Stephanie Seneff supervised and edited. All authors read and approved the final manuscript.
References
American Psychiatric Association, A. (1980). Diagnostic and statistical manual of mental disorders (Vol. 3). Washington, DC: American Psychiatric Association.
Akande, K. E., Doma, U. D., Agu, H. O., & Adamu, H. M. (2010) Major antinutrients found in plant protein sources: their effect on nutrition. Pakistan journal of nutrition, 9(8), 827-832. https://doi.org/10.3923/pjn.2010.827.832.
Bahr, S. M., Tyler, B. C., Wooldridge, N., Butcher, B. D., Burns, T. L., Teesch, L. M.,… & Calarge, C. (2015) Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Translational psychiatry, 5(10), e652-e652. https://doi.org/10.1038/tp.2015.135
Berardelli, A., Currà, A., Fabbrini, G., Gilio, F., & Manfredi, M. (2003) Pathophysiology of tics and Tourette syndrome. Journal Of Neurology, 250(7), 781-787. https://doi.org/10.1007/s00415-003-1102-4
Bernard, B., Stebbins, G., Siegel, S., Schultz, T., Hays, C., & Morrissey, M. et al. (2009) Determinants of quality of life in children with Gilles de la Tourette syndrome. Movement Disorders, 24(7), 1070-1073. https://doi.org/10.1002/mds.22487
Bloch, M., Peterson, B., Scahill, L., Otka, J., Katsovich, L., Zhang, H., & Leckman, J (2006) Adulthood Outcome of Tic and Obsessive-Compulsive Symptom Severity in Children With Tourette Syndrome. Archives Of Pediatrics & Adolescent Medicine, 160(1), 65. https://doi.org/10.1001/archpedi.160.1.65
Bloch, M., & Leckman, J. (2009). Clinical course of Tourette syndrome. Journal Of Psychosomatic Research, 67(6), 497-501. https://doi.org/10.1016/j.jpsychores.2009.09.002
Briguglio, M., Dell’Osso, B., & Porta, M. (2021). Successful nutrition-based approach in Gilles de la Tourette syndrome heralding drug side effects: A case report and short review. Nutrition Clinique Et Métabolisme, 35(3), 231-236. https://doi.org/10.1016/j.nupar.2021.01.111
Canitano, R., & Vivanti, G. (2007). Tics and Tourette syndrome in autism spectrum disorders. Autism, 11(1), 19-28. https://doi.org/10.1177/1362361307070992
Campbell-McBride, N (2020) Gut and Physiology Syndrome: Natural Treatment for Allergies, Autoimmune Illness, Arthritis, Gut Problems, Fatigue, Hormonal Problems, Neurological Disease and More. Chelsea Green Publishing.
Cortese, S., Lecendreux, M., Dalla Bernardina, B., Mouren, M. C., Sbarbati, A., & Konofal, E. (2008). Attention-deficit/hyperactivity disorder, Tourette’s syndrome, and restless legs syndrome: the iron hypothesis. Medical Hypotheses, 70(6), 1128-1132. https://doi.org/10.1016/j.mehy.2007.10.013
Cussotto, S., Strain, C. R., Fouhy, F., Strain, R. G., Peterson, V. L., Clarke, G., … & Cryan, J. F. (2019). Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology, 236(5), 1671-1685. https://doi.org/10.1007/s00213-018-5006-5
Cryan, JF., O’Riordan, KJ., Cowan, CS., Sandhu, KV., Bastiaanssen, TF., Boehme, M. & Dinan,TG. (2019) The microbiota-gut-brain axis. Physiological reviews. https://doi.org/10.1152/physrev.00018.2018
Dan, Z., Mao, X., Liu, Q., Guo, M., Zhuang, Y., Liu, Z., … & Liu, X. (2020). Altered gut microbial profile is associated with abnormal metabolism activity of autism spectrum disorder. Gut Microbes, 11(5), 1246-1267. https://doi.org/10.1080/19490976.2020.1747329
Davis, R. E., & Osorio, I. (1998). Childhood caffeine tic syndrome. Pediatrics, 101(6), e4-e4. https://doi.org/10.1542/peds.101.6.e4
Delaunay-Vagliasindi, S., Seneff, S., & Campbell-McBride, N. (2021). GAPS Nutritional Protocol: How healing the gut removes the basis for all chronic diseases. Journal of Orthomolecular Medicine, 36(3)
Eddy, C., Rickards, H., & Cavanna, A. (2010). Treatment strategies for tics in Tourette syndrome. Therapeutic Advances In Neurological Disorders, 4(1), 25-45.https://doi.org/10.1177/1756285610390261
Evans, J., Seri, S., & Cavanna, A. (2016). The effects of Gilles de la Tourette syndrome and other chronic tic disorders on quality of life across the lifespan: a systematic review. European Child & Adolescent Psychiatry, 25(9), 939-948. https://doi.org/10.1007/s00787-016-0823-8
Garcia-Lopez, R., Perea-Milla, E., Garcia, C. R., Rivas-Ruiz, F., Romero-Gonzalez, J., Moreno, J. L., … & Diaz, J. C. R. (2009). New therapeutic approach to Tourette Syndrome in children based on a randomized placebo-controlled double-blind phase IV study of the effectiveness and safety of magnesium and vitamin B6. Trials, 10(1), 1-12. https://doi.org/10.1186/1745-6215-10-16
Gorman, D. A., Zhu, H., Anderson, G. M., Davies, M., & Peterson, B. S. (2006). Ferritin levels and their association with regional brain volumes in Tourette’s syndrome. American Journal of Psychiatry, 163(7), 1264-1272. https://doi.org/10.1176/ajp.2006.163.7.1264
Grimaldi, B. L. (2002). The central role of magnesium deficiency in Tourette’s syndrome: causal relationships between magnesium deficiency, altered biochemical pathways and symptoms relating to Tourette’s syndrome and several reported comorbid conditions. Medical hypotheses, 58(1), 47-60. https://doi.org/10.1054/mehy.2001.1447
Han, V., Patel, S., Jones, H., & Dale, R. (2021). Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nature Reviews Neurology, 17(9), 564-579. https://doi.org/10.1038/s41582-021-00530-8
Hashemiyoon, R., Kuhn, J., & Visser-Vandewalle, V. (2016). Putting the Pieces Together in Gilles de la Tourette Syndrome: Exploring the Link Between Clinical Observations and the Biological Basis of Dysfunction. Brain Topography, 30(1), 3-29. https://doi.org/10.1007/s10548-016-0525-z
Hienert, M., Gryglewski, G., Stamenkovic, M., Kasper, S., & Lanzenberger, R. (2018). Striatal dopaminergic alterations in Tourette’s syndrome: a meta-analysis based on 16 PET and SPECT neuroimaging studies. Translational Psychiatry, 8(1). https://doi.org/10.1038/s41398-018-0202-y
Johnson, R. J., Gold, M. S., Johnson, D. R., Ishimoto, T., Lanaspa, M. A., Zahniser, R., & Avena, N. M. (2011). Attention-deficit/hyperactivity disorder: is it time to reappraise the role of sugar consumption?. Postgraduate medicine, 123(5), 39-49. https://doi.org/10.3810/pgm.2011.09.2458
Jones, H., Han, V., Patel, S., Gloss, B., Soler, N., & Ho, A. et al. (2021). Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: Transcriptomic data show common enriched innate immune pathways. Brain, Behavior, And Immunity, 94, 308-317. https://doi.org/10.1016/j.bbi.2020.12.035
Kanhere, Yogesh U Rahangdale, Ankita S Bodele, Dipesh I Wadhwani, Abhilasha R Ghoshewar, & Shweta P Karande. (2021). Neurological disorders associated with impaired gut microbiota. GSC Biological And Pharmaceutical Sciences, 015(02), 029-039. https://doi.org/10.30574/gscbps.2021.15.2.0121
Karagiannidis, I., Tsetsos, F., Padmanabhuni, S., Alexander, J., Georgitsi, M., & Paschou, P. (2016). The Genetics of Gilles de la Tourette Syndrome: a Common Aetiological Basis with Comorbid Disorders?. Current Behavioral Neuroscience Reports, 3(3), 218-231. https://doi.org/10.1007/s40473-016-0088-z
Khalifa, N., & Von Knorring, A. (2006). Psychopathology in a Swedish Population of School Children With Tic Disorders. Journal Of The American Academy Of Child & Adolescent Psychiatry, 45(11), 1346-1353. https://doi.org/10.1097/01.chi.0000251210.98749.83
Leckman, J., Zhang, H., Vitale, A., Lahnin, F., Lynch, K., & Bondi, C. et al. (1998). Course of Tic Severity in Tourette Syndrome: The First Two Decades. Pediatrics, 102(1), 14-19. https://doi10.1542/peds.102.1.14
Leeming, E., Johnson, A., Spector, T., & Le Roy, C. (2019). Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients, 11(12), 2862. https://doi.org/10.3390/nu11122862
Liang, H., Sun, X., Ma, A., & Liu, Y. (2015). Evaluation of dietary behavior and nutrient intake in patients with Tourette Syndrome. The FASEB Journal, 29, 911-16. https://doi.org/10.1096/fasebj.29.1_supplement.911.16
Liao, J. F., Cheng, Y. F., Li, S. W., Lee, W. T., Hsu, C. C., Wu, C. C., … & Tsai, Y. C. (2019). Lactobacillus plantarum PS128 ameliorates 2, 5-Dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota–gut-brain-axis. Brain research bulletin, 153, 59-73. https://doi.org/10.1016/j.brainresbull.2019.07.027
Lkhagva, E., Chung, H., Ahn, J., & Hong, S. (2021). Host Factors Affect the Gut Microbiome More Significantly than Diet Shift. Microorganisms, 9(12), 2520. https://doi.org/10.3390/microorganisms9122520
Lloyd-Price, J., Mahurkar, A., Rahnavard, G., Crabtree, J., Orvis, J., Hall AB & Huttenhower, C. (2017) Strains, functions and dynamics in the expanded Human Microbiome Project. Nature, 550(7674), 61-66.
https://doi.org/10.1038/nature23889
Ludlow, A., & Rogers, S. (2017). Understanding the impact of diet and nutrition on symptoms of Tourette syndrome: A scoping review. Journal Of Child Health Care, 22(1), 68-83. https://doi.org/10.1177/1367493517748373
Mohammadi, M., Badrfam, R., Khaleghi, A., Ahmadi, N., Hooshyari, Z., & Zandifar, (2021). Lifetime Prevalence, Predictors and Comorbidities of Tic Disorders: A Population—Based Survey of Children and Adolescents in Iran. Child Psychiatry & Human Development. https://doi.org/10.1007/s10578-021-01186-7
Mondot, S., Lepage, P., Seksik, P., Allez, M., Tréton, X., Bouhnik, Y., … & Marteau (2016). Structural robustness of the gut mucosal microbiota is associated with Crohn’s disease remission after surgery. Gut, 65(6), 954-962. http://dx.doi.org/10.1136/gutjnl-2015-309184
Mueller, N. T., Bakacs, E., Combellick, J., Grigoryan, Z., & Dominguez-Bello, M. G. (2015). The infant microbiome development: mom matters. Trends in molecular medicine, 21(2), 109–117. https://doi.org/10.1016/j.molmed.2014.12.002
Müller‐Vahl, K. R., Buddensiek, N., Geomelas, M., & Emrich, H. M. (2008). The influence of different food and drink on tics in Tourette syndrome. Acta Paediatrica, 97(4), 442-446. https://doi.org/10.1111/j.1651-2227.2008.00675.x
Naureen, Z., Farooq, S., Zahoor, T., & Gilani, S. (2022). Effect of Probiotics on Gut Microbiota and Brain Interactions in the Context of Neurodegenerative and Neurodevelopmental Disorders. In R. Sayyed & M. Khan, Microbiome-Gut-Brain Axis (1st ed., pp. 383-399). Singapore: Springer. Retrieved from https://doi.org/10.1007/978-981-16-1626-6
Planells, E., Lerma, A., Sanchez-Morito, N., Aranda, P., & Llopis, J. (1997). Effect of magnesium deficiency on vitamin B2 and B6 status in the rat. Journal of the American College of Nutrition, 16(4), 352-356. https://doi.org/10.1080/07315724.1997.10718697
Pringsheim, T., Lang, A., Kurlan, R., Pearce, M., & Sandor, P. (2009). Understanding disability in Tourette syndrome. Developmental Medicine & Child Neurology, 51(6), 468-472. https://doi.org/10.1111/j.1469-8749.2008.03168.x
Quagliarello, A., Del Chierico, F., Russo, A., Reddel, S., Conte, G., Lopetuso, L. R.,… & Putignani, L. (2018). Gut microbiota profiling and gut–brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Frontiers in microbiology, 9, 675. https://doi.org/10.3389/fmicb.2018.00675
Robertson, M., Trimble, M., & Lees, A. (1989). Self-injurious behaviour and the Gilles de la Tourette syndrome: a clinical study and review of the literature. Psychological Medicine, 19(3), 611-625. https://doi.org/10.1017/s0033291700024211
Robertson, M. (2008). The prevalence and epidemiology of Gilles de la Tourette syndrome. Journal Of Psychosomatic Research, 65(5), 461-472. https://doi.org/10.1016/j.jpsychores.2008.03.006
Robertson, M., Eapen, V., Singer, H., Martino, D., Scharf, J., & Paschou, P. et al. (2017). Gilles de la Tourette syndrome. Nature Reviews Disease Primers, 3(1). https://doi.org/10.1038/nrdp.2016.97
Rodrigo, L., Huerta, M., & Salas-Puig, J. (2015). Tourette syndrome and non-coeliac gluten sensitivity. Clinical Remission with a Gluten-Free Diet: A Description Case. J Sleep Disord Ther, 4(183), 2167-0277.
Rodrigo, L., Álvarez, N., Fernández-Bustillo, E., Salas-Puig, J., Huerta, M., & Hernández-Lahoz, C. (2018). Efficacy of a Gluten-Free Diet in the Gilles de la Tourette Syndrome: A Pilot Study. Nutrients, 10(5), 573. https://doi.org/10.3390/nu10050573
Scahill, L., Sukhodolsky, D. G., Williams, S. K., & Leckman, J. F. (2005). Public health significance of tic disorders in children and adolescents. Advances in Neurology, 96, 240-248
Schaefer, S. M., Chow, C. A., Louis, E. D., & Robakis, D. (2017). Tic exacerbation in adults with Tourette syndrome: a case series. Tremor and Other Hyperkinetic Movements, 7. https://doi.org/10.7916/D8FF3Z1Q
Silva, R. R., Muñoz, D. M., Daniel, W., Barickman, J., & Friedhoff, A. J. (1996). Causes of haloperidol discontinuation in patients with Tourette’s disorder: management and alternatives. The Journal of clinical psychiatry, 57(3), 129-135.
Singer, H. (2010). Treatment of Tics and Tourette Syndrome. Current Treatment Options In Neurology, 12(6), 539-561. https://doi.org/10.1007/s11940-010-0095-4
Smith, B., & Ludlow, A. (2021). Patterns of Nutritional Supplement Use in Children with Tourette Syndrome. Journal Of Dietary Supplements, 1-16. https://doi.org/10.1080/19390211.2021.1958120
Smith, B., Rogers, S. L., Blissett, J., & Ludlow, A. K. (2019). The role of sensory sensitivity in predicting food selectivity and food preferences in children with Tourette syndrome. Appetite, 135, 131-136. https://doi.org/10.1016/j.appet.2019.01.003
Spadoni, I., Fornasa, G., & Rescigno, M. (2017). Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nature Reviews Immunology, 17(12), 761-773. https://doi:10.1038/nri.2017.100
Stern, E., Silbersweig, D., Chee, K., Holmes, A., Robertson, M., & Trimble, M. et al. (2000). A Functional Neuroanatomy of Tics in Tourette Syndrome. Archives Of General Psychiatry, 57(8), 741. https://doi.org/10.1001/archpsyc.57.8.741
Talari, K., & Goyal, M. (2020). Retrospective studies – utility and caveats. Journal Of The Royal College Of Physicians Of Edinburgh, 50(4), 398-402. https://doi.org/10.4997/jrcpe.2020.409
Worbe, Y., Mallet, L., Golmard, J., Béhar, C., Durif, F., & Jalenques, I. et al. (2010). Repetitive Behaviours in Patients with Gilles de la Tourette Syndrome: Tics, Compulsions, or Both?. Plos ONE, 5(9), e12959. https://doi.org/10.1371/journal.pone.0012959
Wu, C., Wong, L., Hsu, C., Yang, C., Tsai, Y., & Cheng, F. et al. (2021). Randomized Controlled Trial of Probiotic PS128 in Children with Tourette Syndrome. Nutrients, 13(11), 3698. https://doi.org/10.3390/nu13113698
Zhang, X., Zhang, D., Jia, H., Feng, Q., Wang, D., Liang, D., … & Wang, J. (2015). The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nature medicine, 21(8), 895-905. https://doi.org/10.1038/nm.3914
Zhao, H., Luo, X., Shi, Y., Li, J., Pan, F., & Ren, R. et al. (2020). The Efficacy of Fecal Microbiota Transplantation for Children With Tourette Syndrome: A Preliminary Study. Frontiers In Psychiatry, 11. https://doi.org/10.3389/fpsyt.2020.554441
Zimmer, M. H., Hart, L. C., Manning-Courtney, P., Murray, D. S., Bing, N. M., & Summer, S. (2012). Food variety as a predictor of nutritional status among children with autism. Journal of autism and developmental disorders, 42(4), 549-556. https://doi.org/10.1007/s10803-011-1268-z
Zou, L. P., Wang, Y., Zhang, L. P., Zhao, J. B., Lu, J. F., Liu, Q., & Wang, H. Y. (2011). Tourette syndrome and excitatory substances: is there a connection?. Child’s Nervous System, 27(5), 793-802. https://doi.org/10.1007/s00381-010-1318-4


