.
Introduction
In recent years, various environmental adaptations have manifested in response to local, regional and global changes in the climate, such as changes in rainfall patterns, surface and air temperature, winds and ocean currents. These environmental adaptations are thought to be a primary contributor to the emergence of new viral variants. These adaptations are predicted to affect viral infections at three levels: change in transmission patterns, the ecology of the host, and socioeconomical changes influencing host populations. Climate change may influence epidemiological outcome as well as the morbidity and mortality associated with a particular viral infection.
Therefore, the increased risk for those infection diseases urges us to consider a valid strategy for prevention of infection, especially for those who have underlying diseases, or elders in general. As one of the effective measures for the prevention of infectious diseases, boosting the immune strength of human biological protections has been thought to be essential. There are certain known strategies to improve immune strength, like moderate exercise (Suzuki K, 2004), stress relief (Murakami, Kamoshita, Sawada, & Katsura, 1989), and dietary habits to optimize the intestinal environment. Fermented foods have been a recent focus of attention for their effect on the gut immune system (Hosoi, 2003; Wastyk et al., 2021), antioxidant effect, and anti-inflammation effect (Miyamoto, Noda, Ohya, & Kamada, 2000; Nakamura & Esaki, 2013; Suzuki S, 2004). Particularly foods containing lactic acid bacteria (LAB) as an active ingredient have been reported to enhance innate and adaptive immunity, prevent gastric mucosal lesion development, and improve defense against intestinal pathogens (Hachimura, 2007; Miyoshi, 2020; Tategaki, 2019)
OM-X,he test product in this research, is a fermented supplement developed by Dr. Iichiro Ohira. OM-X has been sold for more than 30 years in 10 countries in the world including Japan. OM-X contains 12 kinds of lactic acid bacteria, bifidobacteria, oligosaccharide, dietary fiber, and lipids (short-chain fatty acids). Thus far, OM-X has been reported to have beneficial effects on bone and oral health (Kawakami et al., 2003; Hashim, Rahman, & Philip, 1999), as well as physical capabilities (Kawakami et al., 1998). In this report, we deliver the result of our study on OM-X’s effects on human immune modulation through the randomized, placebo-controlled, double-blind, parallel-group trial run for 12 weeks.
Materials and Methods
Study Design
A randomized, placebo-controlled, double-blind paralleled design was implemented from August to November 2021 at Japan Clinical Trial Association; JACTA, Tokyo. This trial was registered at UMIN Clinical Trial Registry as UMIN000045192.
Participants
Healthy subjects participated in the present study. All subjects were public volunteers who enrolled in the monitor bank of InCROM Inc. (Nishiwaki, Hyogo), recruited from July through August 2021.
The inclusion criteria were as follows: (1) healthy Japanese males and females from 40 to 59 years of age; (2) a self-reported feeling of unwellness at the time of study enrollment and/or predisposition to colds. The exclusion criteria were as follows: (1) chronic fatigue or diagnosed chronic fatigue syndrome; (2) serious cerebrovascular disease, heart disease, liver disease, renal disease, gastrointestinal disease, psychiatric disease, or designated infectious disease; (3) receiving treatment (hormone replacement therapy, drug therapy, exercise therapy, diet therapy, etc.) or those who are judged to be in need of treatment at the time of receipt of informed consent; (4) consistent use of supplements and/or functional foods affecting immunity, including food for specified health uses (FOSHU); (5) currently using drugs for food allergies; (6) have a history of alcohol or drug dependence; (7) a history of recent cigarette use within the past year; (8) are pregnant or planning to become pregnant during the study, or breastfeeding; (9) have participated in other clinical trials one month prior to receipt of informed consent, or those who plan to enroll in other clinical trials during this trial; (10) plan for vaccination against COVID-19 within 7 days before the visit; (11) are judged as unsuitable for the study by the principal investigator.
Randomization
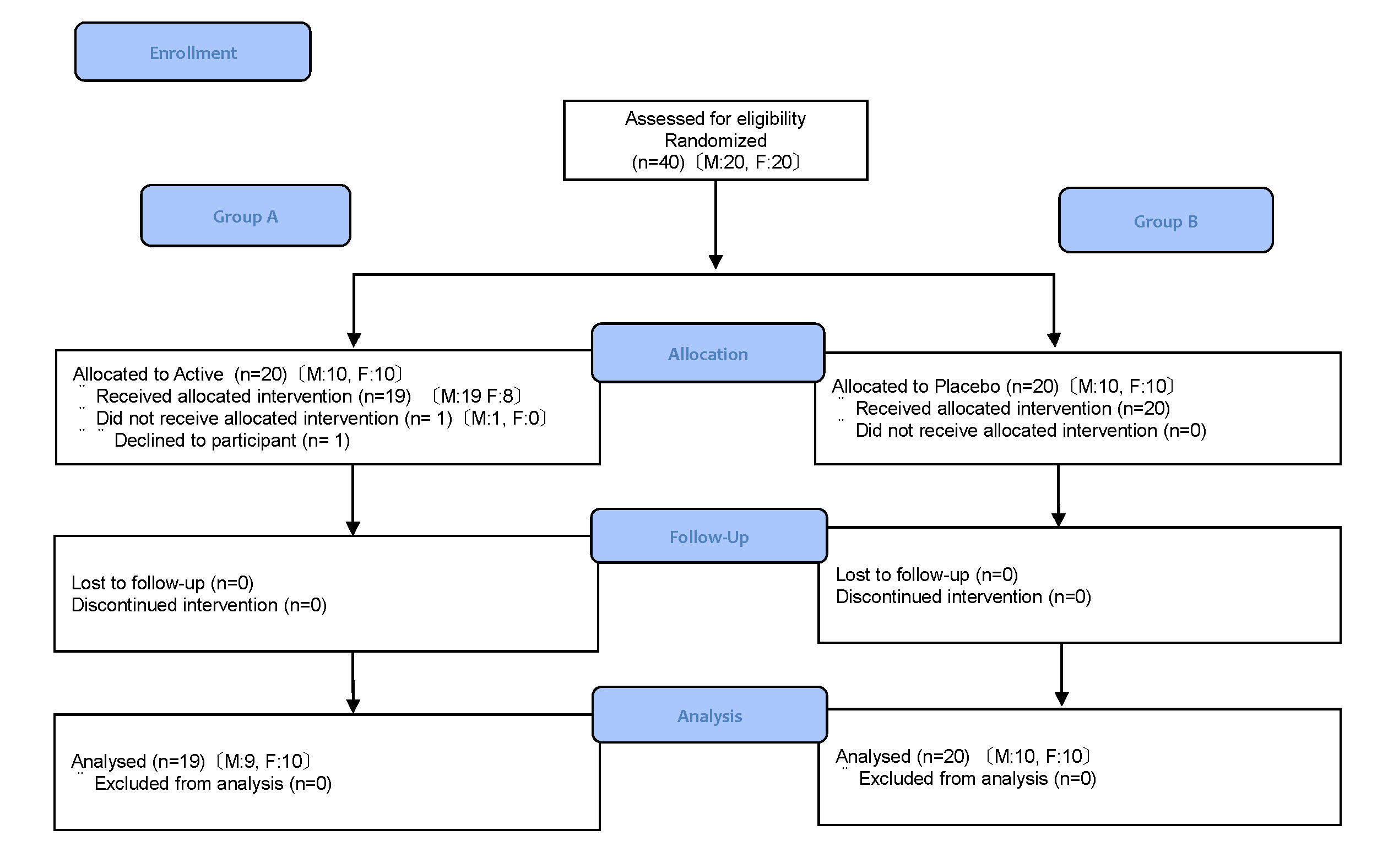
According to inclusion/exclusion criteria, 40 subjects were selected and sequentially allocated to two groups of 20 each using a random number table: Group A and B. Among those, one subject in Group A declined to participate in the study, and the remaining 39 subjects launched allocated intervention. Background factors such as gender and age were taken into consideration to avoid biased distribution. The allocation list was sealed and strictly controlled until the end of the study. Subjects in Group A received the test sample (Active) and subjects in Group B received Placebo. As shown in Figure 1.
Figure 1. Flow diagram of subject disposition
Experimental Groups and Test Foods
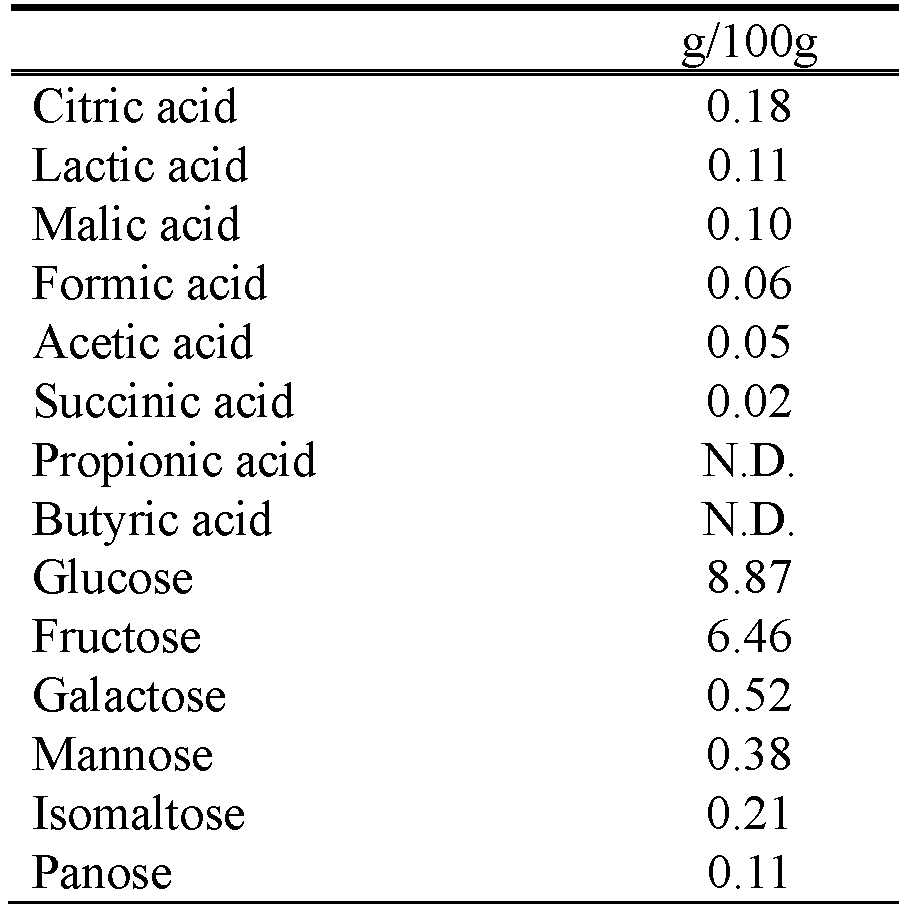
Group A, the active group, received OM-X. Group B received placebo. OM-X is made of fermented extracts from several kinds of Japanese plants with 12 kinds of lactic acid bacteria (hereinafter called LAB) and bifidobacteria such as TH10. OM-X also includes fulvic acid, D-amino acids, L-amino acids, melanoidin, polyphenols, organic acids, fatty acids, vitamins, minerals, dietary fibers, and oligosaccharides. . The placebo received by Group B was made primarily from safflower oil. The amount of daily intake was 3 softgel capsules (1 capsule weighs 580 mg) for each group. Both active and placebo foods, prepared by BIOBANK Co., Ltd., were indistinguishable in shape, color, or taste, and were managed by identification marks. . Table 1 and Table 2 show the ingredients contained in OM-X production and composition of organic acids and carbohydrates in OM-X.
Table 1. Composition of food materials for making OM-X

Table 2. Composition of organic acids and carbohydrates in OM-X
Experimental Procedures
Subjects in both the placebo and experimental groups received three oral softgel capsules once a day for 12 weeks. All subjects were instructed to: take the assigned capsules daily; maintain their usual lifestyle and habits; avoid excessive amounts of food, drink, or alcohol; avoid any medicine or FOSHU (food for specified health uses) with an immunomodulating effect; maintain a daily record of their physical condition, sleeping, appetite, and exercise; and send the diary once a week to the study coordinator.
Outcome
The objective of this study was to explore the potential immunomodulating activity of OM-X in healthy subjects with a predisposition to cold-like symptoms. The following assessments were administered to subjects: the health-related quality of life (HRQOL) scale; the Japanese version of SF-8 health survey; and a questionnaire evaluating subjects’ physical condition (Table 4). Furthermore, the safety of the test foods (OM-X and placebo) was evaluated as the secondary outcome. The SF-8 was administered pre- and post-intervention. The questionnaires for assessing subjects’ physical condition and the safety of the test foods were collected as a diary during the study.
The Japanese version of the SF-8 (© QualityMetric Inc., Fukuhara, S) is a comprehensive scale that is suitable for the continuous measurement of HRQOL in healthy individuals. The subscales consist of physical functioning; PF, role physical; RP, body pain; BP, general health; GH, vitality; VT, social functioning; SF, role emotional; RE, and mental health; MH (Fukuhara & Suzukamo, 2004, 2019a; Fukuhara & Suzukamo, 2005). The two summary scores are physical component summary, PCS; and mental component summary, MCS (Fukuhara & Suzukamo, 2004, 2019b). The higher the score for all 10 items combined, eight subscales plus two summary scores, the higher the QOL.
Physical Condition
The questionnaire assessing subjects’ physical condition, included the following symptom inventory: runny nose, nasal congestion, sore throat, cough, lassitude, articular pain, chill, and the sum of all symptoms (Kokubo et al., 2020; Kondo, 2021; Shibata, 2016). Subjects were instructed to report daily symptoms marked with a circle or cross, then the total number of days with symptoms during phase 1 (7 days immediately after the start of the study; 1-7 days later) and phase 2 (7 days immediately before the end of the study; 78-84 days later) were counted. The fewer days, the better physical condition.
Safety
The safety of the test food was evaluated utilizing a written questionnaire during the study. Any unexpected medical occurrence during the study period, defined an adverse event, does not necessarily have to have a causal relationship with the test food. The principal investigator determined the relations between cause and consequence of the adverse events.
Data Analysis
An intention-to-treat (ITT) analysis was adopted, and no sample size calculation was applied. All statistics were expressed as mean ± standard deviation (SD). Chi-square test and Student’s t-test were used to compare the subject’s backgrounds between groups. As for scores, the paired t-test was used for intragroup analysis, and Student’s t-test was used for intergroup comparisons to quantify the amount of change before and after the intervention. Multiplicity was not adjusted for. Statistical analyses were performed using Excel Tokei 3.23 (BellCurve) and Statcel 4 (Yanai, 2015). The level of significance was set to p=0.05 for the two-sided tests.
Results
Subjects
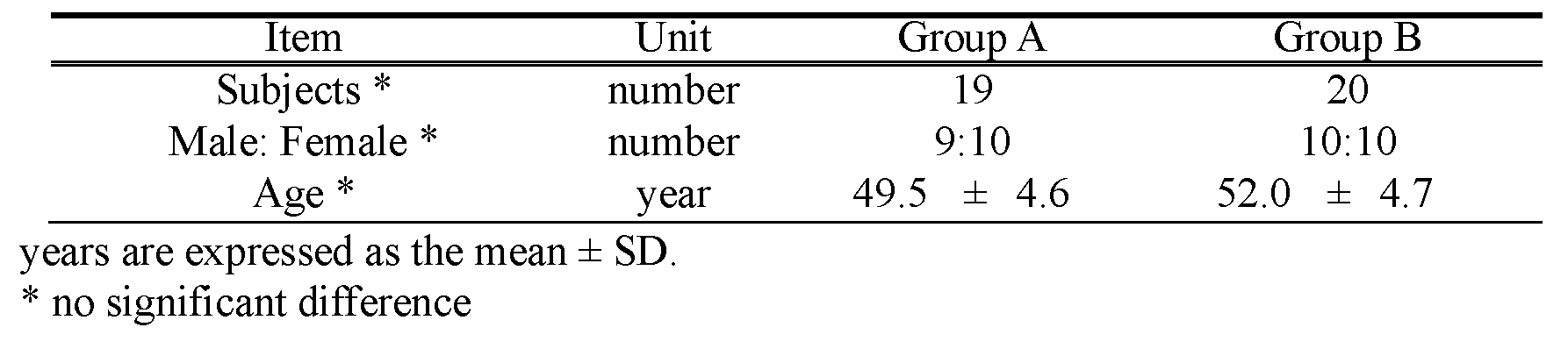
Of the 40 subjects who enrolled, 39 completed the study. There was a high level of adherence to the study protocol, with all subjects consuming their assigned test food. The data obtained from 39 subjects (Group A; 19, Group B; 20) were used for efficacy analysis (Figure 1). The subjects were 50.7 ± 4.8 years of age. There were no significant differences in gender ratio or age at baseline between groups (Table 3).
Table 3. Subject Demographics
Result of Subjective Evaluation
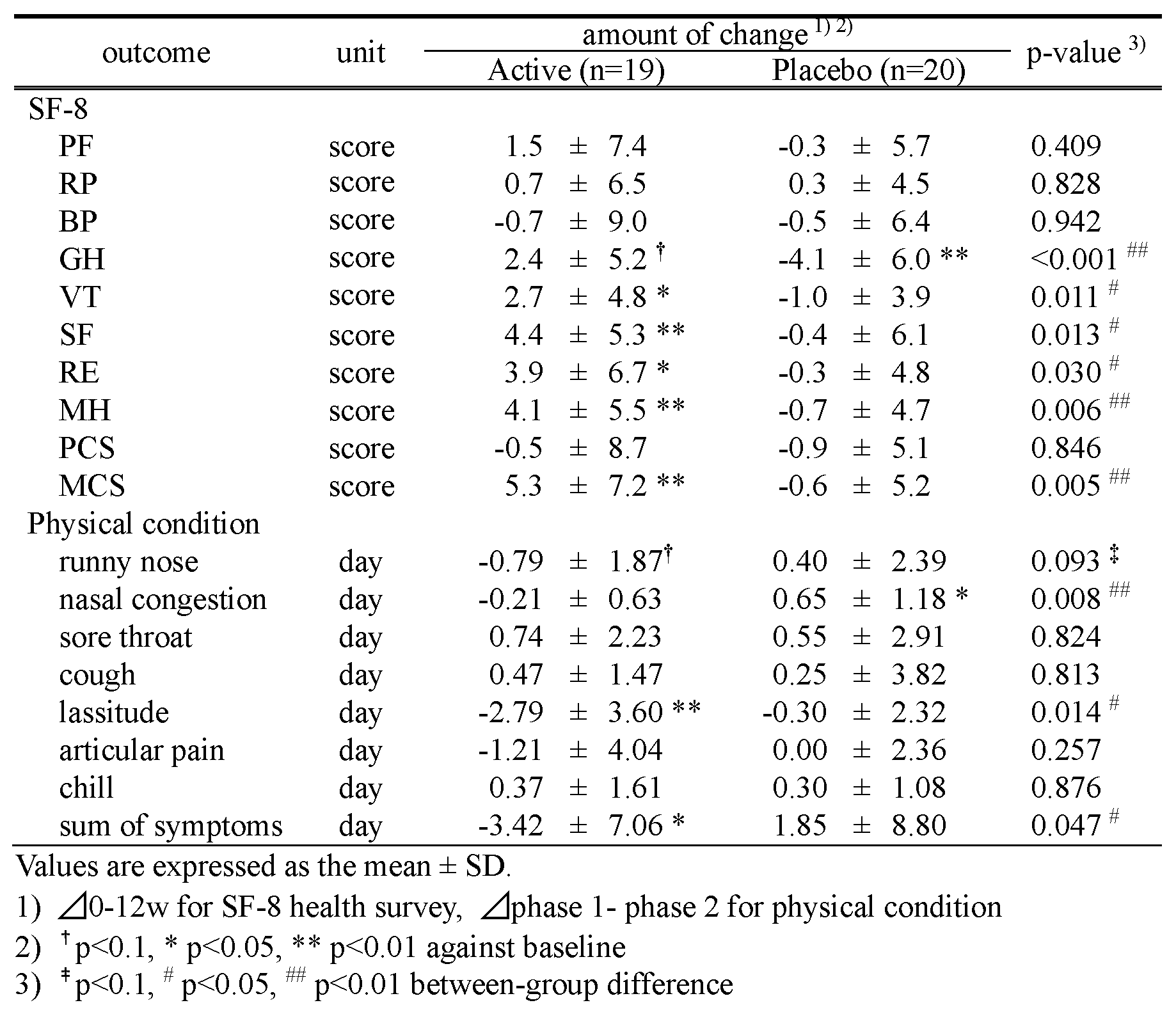
Table 4. indicates the results of SF-8 and physical condition. As for SF-8, 5 items (VT, SF, RE, MH, MCS) out of 10 increased significantly in Active, though the Placebo showed a significant decrease in GH. In addition, intergroup analysis depicted significant differences in 6 items (GH, VT, SF, RE, MH, and MCS). With reference to physical condition, lassitude and sum total of symptoms in Active, and nasal congestion in Placebo showed significant differences after 12 weeks of ingestion. There were significant differences between the two groups in nasal congestion, lassitude, and the sum total of symptoms.
Table 4. Results of subjective evaluation
Adverse Events
As for Active group, the number of days with a sore throat, cough, and chills increased slightly after 12 weeks, though the change in individual subjects was minimal and resolved. Thus the principal investigator determined that there was likely no causal relationship with the test food. No other adverse events occurred, and no clinical side effects were observed.
Discussion
The present study demonstrated that the use of OM-X, containing plant fermentation extract, LAB, and bifidobacterial improves human immune function when used consistently for 12 weeks. The ingestion of 3 capsules of OM-X per day significantly improved 6 items (GH, VT, SF, RE, MH, and MCS) in the HRQOL questionnaire. In addition, there was a significant improvement in nasal congestion, lassitude, and overall physical health subjects receiving OM-X. OM-X appeared to be safe with no signs of adverse effects.
In this study, we attempted to verify the immunomodulating effect of the ingestion of OM-X. Our metabolome analysis confirmed that the plant extract made through a multi-year fermentation process using LAB and bifidobacteria contains hundreds of low-molecular compounds including proteins, peptides, organic acids, short-chain fatty acids, dietary fiber, and polyphenols in the food capsules (data not shown). It is reported that the ingestion of the OM-X extract by type I allergy mice had been proven to be effective in inhibition of RBL-2H3 cells degranulation and PCA (the passive cutaneous anaphylaxis) reaction at the same time (Itoh et al., 2015). This study indicated that the ingestion of the OM-X had promoted the production of nitrogen monoxide, IL-6, and TNF-α within the organism, and activation of macrophage cells (Wakame et al., 2017). The LAB and the bifidobacteria contained in OM-X are representative examples of probiotics (Fuller, 1989) and are reported to be effective in the improvement of intestinal flora balance, prevention of virus infection (Yasui, 2010), and coordination of the immune system (Yasui, Shida, Matsuzaki, & Yokokura, 1999). LAB are also known for their positive effects on immunostimulatory activities (Morimoto, 2005; Perdigon, Alvarez, Rachid, Agüero, & Gobbato, 1995), anti-allergic properties (Majamaa & Isolauri, 1997; Suzuki S et al., 2020), balance ability between Th1 and Th2 (Hachimura, 2007; Sashihara, 2013), and antibiotic activities due to activation of pDCs (Tanaka, 2015). As well, TH10, a prime component of the lactic acid bacteria in OM-X has been reported to help activation of macrophages (Itoh, 2012). In addition, antioxidative substances such as polyphenol contained in OM-X has been reported to be effective in suppressing the oxidative stress which is associated with various diseases and their progressions (Tanaka et al., 2016). In this study, the original lactic acid bacterium, TH10, and other bifidobacteria contained in OM-X together were thought to bring positive effects on immunoregulation via maintaining a balance between Th1 and Th2 as a key to immunological responses and activating macrophage cells and dendritic cells..
It is assumed that the long-term fermentation and aging process of the extract helped produce melanoidin as a result of a Maillard reaction between sugar and amino acids. Melanoidin has been shown to suppress the degranulation of granulocytes (neutrophils, basophils, and eosinophils) and mast cells and thus reduce allergy-related symptoms such as cough and runny nose (Itoh, 2015).
Conclusion
The results of the study suggested that the OM-X, a plant fermentation extract containing lactic acid bacteria and bifidobacteria, and its ingestion for 12 weeks might have an immunomodulating effect on healthy subjects with a feeling of unwellness and/ or preposition to colds. OM-X was found to be safe for consumption.
Conflict of Interest
All parts of this study were funded by BIOBANK Co., Ltd. MT is an executive officer of BIOBANK Co., Ltd. The other authors declare that the study was conducted in the absence of any financial relationships that could be interpreted as a conflict of interest.
Ethics Statements
The authors ensure that the current clinical trial has been carried out in accordance with the ethical principles of the declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. The protocol was approved by the Institutional Review Board of Pharmaceutical Law Wisdoms, Tokyo, and was conducted in compliance with the protocol. Written informed consent was obtained from all subjects prior to enrollment.
Funding
This work was supported by BIOBANK Co., Ltd., Okayama, Japan.
References
Fujii, T., Ogino, S., Arimoto, H., Irifune, N., Iwata, N., Ookawa, I., Kikumori, H., Seo, R., Takeda, M., Tamaki, A., Baba, K., & Nose, M. (2007). Correlation between the SF-8 health status questionaire and JRQLQ patients with Japanese cedar pollinosis. Japanese Journal of Allergology. 56(2): 109-117. https://doi.org/10.15036/arerugi.56.109
Fukuhara, S. & Suzukamo, Y. (2004, 2019a). Manual of the SF-8 Japanese version. iHope International K.K. Kyoto.
Fukuhara, S. & Suzukamo, Y. (2004, 2019b). Manual of the SF-36v2 Japanese version. iHope International K.K., Kyoto.
Fukuhara, T. & Suzukamo, Y. (2005). Health-related quality of life scales-SF-8 and SF-36. Journal of Clinical and Experimental Medicine. 213: 133-136. http://www.pieronline.jp/content/article/0039-2359/213020/133
Fuller, R. (1989). Probiotics in man and animals. The Journal of applied bacteriology. 66(5): 365-378. https://doi.org/10.1111/j.1365-2672.1989.tb05105.x
Hachimura, S. (2007). Immune-modulation by lactic acid bacteria. Japanese Journal of Lactic Acid Bacteria. 18(2): 54-57. https://doi.org/10.4109/jslab.18.54
Honaga, K., Yamada, S., & Liu, M. (2006). Use of the SF-8 to assess Health-Related Quality of Life (HRQOL) in Elderly Patients with SMON (Subacute Myelo-Optico-Neuropathy). The Japanese Journal of Rehabilitation Medicine. 43(11): 762-6. https://doi.org/10.2490/jjrm1963.43.762
Hosoi, T. (2003). Probiotic effects of bacillus subtilis (natto). Journal of the brewing society of Japan. 98(12): 830-839. https://doi.org/10.6013/jbrewsocjapan1988.98.830
Itoh, T., Miyake, Y., Onda, A., Kubo, J., Ando, M., Tsukamasa, Y., & Takahata, M. (2012). Immunomodulatory effects of heat-killed Enterococcus faecalis TH10 on murine macrophage cells. Microbiology Open. 1(4): 373-380. https://doi.org/10.1002/mbo3.41
Itoh, T., Miyake, Y., Kasashima, T., Shimomiya, Y., Nakamura, Y., Ando, M., Tsukamasa, Y., & Takahata, M. (2015). OM-X®, Fermented vegetables extract suppresses antigen-stimulated degranulation in rat basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Natural product communications. 10(9): 1597-1601. https://doi.org/10.1177/1934578X1501000928
Itokazu, M., Ito, Y., Takigami, I., Suzuki, A., Ohno, K., & Ogawa, H. (2007). Evaluation of Quality of Life (SF-8) Following Treatment with Tacrolimus in Patients with Rheumatoid Arthritis. Japanese Journal of Rheumatism and Joint Surgery. 26(4): 403-11. https://doi.org/10.11551/jsjd1982.26.403
Jounai, K., Ikado, K., Sugimura, T., Ano, Y., Braun, J., & Fujiwara, D. (2012). Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS One. 7(4): e32588. https://doi.org/10.1371/journal.pone.0032588
Kaida, K., Shimadu, K., & Fukumitu, H. (2017). Arthritis prevention effect and commercialization of Shodoshima-producing olive-derived fruit extract. Seibutsu-kogaku Kaishi. 95(6): 315-317. https://cir.nii.ac.jp/crid/1520009407075811840
Kawakami, M., Ohhira, L., Araki, N., Inokihara, K., Mtsubara, T., & Iwasaki, H. (1998). The influences on the VO2 max of athlete with taking lactic acid bacteria (OM-X). Bulletin of Kurashiki University of Science and the Arts. 3: 131-144. https://jglobal.jst.go.jp/detail?JGLOBAL_ID=200902152593747154
Kawakami, M., Ohhira, L., Araki, N.,Inokihara, K., Iwasaki, H., & Matsubara, T. (2003). The influence of lactic acid bacteria (OM-X) on bone structure. Journal of applied nutrition. 53: 1-6. https://www.researchgate.net/publication/285767600_The_influence_of_lactic_acid_bacteria_OM-X_on_bone_structure
Kokubo, T., Wakai, S., Fujiwara, D., Kanauchi, O., Jounai, K., Ichikawa, H., Takuma, M., Kanaya, Y., & Shiraoka, R. (2020). Lactococcus lactis Strain Plasma Improves Subjective Physical State and Presenteeism: A Randomized, Open-Label Crossover Study among Healthy Office Workers. Preventive nutrition and food science. 25(2): 140-145. https://doi.org/10.3746/pnf.2020.25.2.140.
Kondo, S. (2021). Effects of the Food Containing EGCG and L-Theanine from green tea on immune responses and maintenance of physical conditions; a randomized, double-blind, placebo-controlled, parallel-group study. Japanese pharmacology & therapeutics. 49(11): 1937-1948. http://www.pieronline.jp/content/article/0386-3603/49110/1937
Majamaa, H. & Isolauri, E. (1997). Probiotics: a novel approach in the management of food allergy. The Journal of allergy and clinical immunology. 99(2): 179-185. https://doi.org/10.1016/s0091-6749(97)70093-9
Miyamoto, Y., Noda, H., Ohya, H., & Kamada, H. (2000). SOD-like activities of rice bran extracts after fermentation. Nippon Shokuhin Kagaku Kogaku Kaishi. 47(3): 214-219. https://doi.org/10.3136/nskkk.47.214
Miyoshi, M. (2020). The study for immune-modulating effect of lactic acid bacteria. Milk Science. 69(3): 187-191. https://doi.org/10.11465/milk.69.187
Monoi, N. & Sano, T. (2019). The sleep improving food material “Japanese Sake Yeast” and its application to “Foods with Function Claims”. Oleoscience. 19(7): 291-297. https://doi.org/10.5650/oleoscience.19.291
Morimoto, K., Takeshita, T., Nanno, M., Tokudome, S., & Nakayama, K. (2005). Modulation of natural killer cell activity by supplementation of fermented milk containing Lactobacillus casei in habitual smokers. Preventive medicine. 40(5): 589-594. https://doi.org/10.1016/j.ypmed.2004.07.019
Murakami, M., Kamoshita, I., Sawada, S., & Katsura, T. (1989). Stress-induced immunological modulation of defence mechanism: studies in our department (stress and immunity). Japanese Journal of Psychosomatic Medicine. 29(2): 123-131. https://doi.org/10.15064/jjpm.29.2_123
Nagura, H. (1987). Defense mechanism in the intestinal mucosa. KAGAKU TO SEIBUTSU. 25(9): 566-574. https://doi.org/10.1271/kagakutoseibutsu1962.25.566
Nakamura, Y. & Esaki, H. (2013). Changes of the ingredients and the functions of foodstuffs by fermentation; in the case of dark tea and soybean products. Journal of the brewing society of Japan. 108(1): 16-24. https://doi.org/10.6013/jbrewsocjapan.108.16
Nishimura, T. & Ohta, A. (1999). A critical role for antigen-Specific Th1 Cells in acute liver injury in Mice. The Journal of immunology: official journal of the American Association of Immunologists. 162(11): 6503-6509. https://www.jimmunol.org/content/162/11/6503.short
Ono S. & Kabashima, K. (2016). The role of dendritic cells and macrophages in the skin immunity. Japanese Journal of Clinical Immunology. 39(5): 448-454. https://doi.org/10.2177/jsci.39.448
Perdigon, G., Alvarez, S., Rachid, M., Agüero, G., & Gobbato, N. (1995). Immune system stimulation by probiotics. Journal of dairy science. 78(7): 1597-1606. https://doi.org/10.3168/jds.S0022-0302(95)76784-4
Sashihara, T. (2013). Effects of lactic acid bacteria on immune regulatory activities. Journal of Intestinal Microbiology. 27(4): 197-202. https://doi.org/10.11209/jim.27.197
Sato, K. (2016). Dendritic cell subsets and function. Japanese Journal of Allergology. 65(1): 11-16. https://doi.org/10.15036/arerugi.65.11
Shibata,T., Kanayama, M., Haida, M., Fujimo, S., Oroguchi, T., Sata, K., Mita, N., Kutsuzawa, T., Ikeuchi, M., Kondo, M., Naito, K., Tsuda, M., Nishizaki, Y., & Ishii, N. (2016). Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. Journal of Functional Foods. 24: 492-500. https://doi.org/10.1016/j.jff.2016.03.035
Siegal, FP., Kadowaki, N., Shodell, M., Fitzgerald-Bocarsly, PA., Shah, K., Ho, S., Antonenko, S., & Liu YJ. (1999). The nature of the principal type 1 interferon-producing cells in human blood. Science. 284(5421): 1835-1837. https://doi.org/10.1126/science.284.5421.1835.
Suzukamo, Y. (2002). Characteristic and application of SF-36 to healthcare research. The Journal of Japanese Society of Lumbar Spine Disorders. 8(1): 38-43. https://doi.org/10.3753/yotsu.8.38
Suzuki, K. (2004). Exercise and immunity. Japanese Journal of Complementary and Alternative Medicine. 1(1): 31-40. https://doi.org/10.1625/jcam.1.31
Suzuki, S. (2004). About TNF-α activity of fermented plant foods. Journal of Japan Health Medicine Association. 13(3): 72-73. https://doi.org/10.20685/kenkouigaku.13.3_72
Suzuki, S., Kubota, N., Kakiyama, S., Miyazaki, K., Sato, K., & Hariima-Mizusawa, N. (2020). Effect of Lactobacills plantarum YIT 0132 on Japanese cedar pollinosis and regulatory T cells in adults. Allergy. 75(2): 453-456. https://doi.org/10.1111/all.14003
Tanaka, K., Suzuki, H., Kanayama, M., Fujii, T., Fujiwara, D., Nozawa, H., & Sugihara, H. (2015). The safety evaluation of long-term or excessive intake of the beverage containing lactococcus lactis subsp. lactis JCM 5805 and resistant maltodextrin; a randomized, double-blind, placebo-controlled, parallel-group trial. Japanese pharmacology & therapeutics. 43(10): 1465-1472. https://www.pieronline.jp/content/article/0386-3603/43120/1711
Tanaka, Y., Ishii, S., Asagiri, K., Fukahori, S., Saikusa, N., Hashizume, N., Yoshida, N., Komatsuzaki, N., Masui, D., Higashidate, N., & Yagi, M. (2016). Oxidative stress and Antioxidant. Journal of Japanese Society for Parenteral and Enteral Nutrition. 31(1): 3-12. https://doi.org/10.11244/jspen.31.3
Tategaki, A. (2019). Lactic acid bacteria and human health. Comprehensive Medicine. 17(1): 8-19. https://doi.org/10.32183/ifcm.17.1_8
Umegaki, K. (2019). Safety and effectiveness of food with health claims and its effective use. The Japanese Journal of Nutrition and Dietetics. 77(3): 67-75. https://doi.org/10.5264/eiyogakuzashi.77.67
Wakame, K., Nakata, A., Sato, K., Mihara, Y., Takahata, M., Miyake, Y., Okada, M., Shimomiya, Y., & Komatsu, K. (2017). Fermented vegetable and fruit extract (OM-X) stimulates murine gastrointestinal tract cells and RAW264.7 cells in vitro and regulates liver gene expression in vivo. Integrative Molecular Medicine. 4: 1-5. https://doi.org/10.15761/IMM.1000266
Ware Jr., JE. & Sherboune CD. (1992). The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Medical care. 30(6): 473-483. https://www.jstor.org/stable/3765916
Wastyk, HC., Fragiadakis, GK., Perelman, D., Dylan, D., Merrill, BD., Yu, FB., Top, M., Gonzalez, CG., Treuren, WV., Han, S., Robinson, JL., Elias, JE., Sonnenburg, ED., Gardner, CD., & Sonnenburg, JL. (2021). Gut-microbiota-targeted diets modulate human immune status. Cell. 184(16): 4137-4153. https://doi.org/10.1016/j.cell.2021.06.019
Yamaguchi, A., Togashi, Y., Koda, T., & Nishimura, T. (2005). Development of DNA array filter useful for the analysis of Th1/Th2 balance. Japanese Journal of Clinical Immunology. 28(2): 86-91. https://doi.org/10.2177/jsci.28.86
Yamashita, M. (2011). Epigenetics of Th2 cell differentiation and functional maintenance that control allergic reaction. Chiba medical journal. 87: 203-208. https://opac.ll.chiba-u.jp/da/curator/900093498/
Yasui, H., Shida, K., Matsuzaki, T., & Yokokura, T. (1999). Immunomodulatory function of lactic acid bacteria. Antonie Van Leeuwenhoek. 76(1-4): 383-389. https://doi.org/10.1023/A:1002041616085
Yasui, H. (2010). Immunomodulatory effect and protective function against virus infection of probiotics in fermented milk. Milk Science. 59(3): 255-263. https://doi.org/10.11465/milk.59.255
Yoshii, K., Hosomi, K., & Kunisawa, J. (2022). Microbial metabolites for the control of host immunity in the gut. Journal of Intestinal Microbiology. 36(1): 1-11. https://doi.org/10.11209/jim.36.1


