.
The Deadliest Psychiatric Disorder
Every 62 minutes, someone in the United States dies as a direct result of an eating disorder (Eating Disorders Coalition, 2016). That fatality is most likely linked to Anorexia Nervosa (AN). As not only the deadliest eating disorder, but the mental illness with the highest mortality rate, Anorexia Nervosa is a serious epidemiological crisis. AN is officially distinguished by DSM-V criteria by an inability and refusal to maintain an appropriate body weight based on a distorted body image and an intense fear of “fatness” with restriction and avoidance of food in order to prevent weight gain. Individuals with AN are often in denial of their illness severity and engage in ongoing self-destructive behaviors including starvation and over-exercising. A purging subcategory of AN is generally differentiated by engagement in episodes of binge eating followed by self-induced vomiting or laxative use (Frank, 2014).
Despite drawing greater attention in recent decades, AN is not a modern illness, with anecdotal reports from the Middle Ages and its first medical descriptions in 1689 (Arnold, 2016). The lifetime prevalence of AN remains stubbornly fixed at around 1%, with no sign of retreat. Although most common in young females, AN can be found in males and females, children, adolescents, adults, elderly adults, in both developing and affluent countries, and independent of time or ecology. Still, mortality among females age 15 to 24 years old is most distressing, with a risk of death ten times that of their peers (Eating Disorders Coalition, 2016).
It is estimated that fewer than a third of individuals with eating disorders seek and receive treatment, potentially a response to a paucity of successful results (Eating Disorders Coalition, 2016). A review of epidemiological data from 119 studies revealed poor global outcomes in AN patients, with a significant rise in the mortality rate over time indicated by an 18-fold increase over a 15-year period. Data from the study suggested that fewer than 50% of individuals treated for AN fully recover, only a third achieve some symptom improvement, and at least 20% continue in persistent illness (Steinhausen, 2002).
Eating disorder research in the latter half of the 20th century was focused on multicomponent therapies in an attempt to factor in both developmental and biological components. Individual variations in endocrine and emotional characteristics have led to multiple etiological theories and many clinical treatment approaches. Behavioral, chemical, and even surgical interventions for AN have proven mostly ineffective and even unsafe. As a complex and multifactorial disorder with genetic, biological, environmental, and social components, exhaustive clinical research on AN has continued for decades with little progress. Outcome measures for AN have not shown improvement over the last 50 years. One thing is clear: treatment is not simple or straightforward; “Just eat and gain weight” does not work. Many patients do not feel capable of choosing to get well and remain entrenched in deep-seated behaviors leading to cycles of relapse that cause further physical deterioration in a vicious, self-sustaining pattern. At least 15 to 25% of treated patients relapse within two years. The devastating reality is that many adults with Anorexia Nervosa will die as a result of this chronic course, whether through direct effects of malnutrition or by suicide. At least a quarter of patients suffering with AN will also endure psychiatric comorbidities including anxiety and depression, obsessive-compulsive disorder, and substance-abuse issues. In fact, suicide risk in AN patients is at least 1.5 times that of depression alone and recovery is strongly tied to the presence and severity of comorbid conditions (Beumont et al., 2005; Steinhausen, 2002).
Regardless of overwhelming evidence defining Anorexia Nervosa as a multifaceted disorder that is unresponsive to medications, common healthcare practices continue to employ pharmacotherapy as a first-line treatment strategy. A 2016 investigation by Garner, et al., of 500 hospitalized eating disorder patients reported that 86% were taking one or more of 41 different medications. Of those taking medication, 47% were prescribed at least two, 25% three or more, and 11% four or more (Garner et al., 2016). Numerous clinical trials demonstrate that antidepressants and antipsychotics are ineffective for AN. Psychotropic drugs are frequently prescribed to eating disorder patients despite any substantial data demonstrating their efficacy. An assessment of medication use in 525 women with AN reflected a 10% increase in the use of atypical antipsychotics between 1997 and 2009 (Fazeli et al., 2012).
Treatment avoidance and resistance are frequent companions to AN, with refusal and dropout rates for pharmaceutical trials ranging from 40 to 95%. Within clinical settings, typical failure rates are estimated between 20 and 50% for inpatient and 25 to 75% in outpatient settings. The majority of patients abandon treatment based on dissatisfaction with pharmaceutical therapies, strongly indicating that medication alone is not a valid intervention. A literature review of 71 studies conducted between 1990 and 2013 on treatment resistance in AN suggest four main contributors: denial of illness, lack of motivation to change, maintenance difficulties, and incompatible therapeutic relationships; therefore, both lack of patient cooperation and ineffective interventions oppose progress. Illness severity is also a major predictor of treatment success (Abbate-Daga, 2013).
While imbalanced neurotransmitters, the primary target of pharmacological treatments, are certainly critical components in the etiology of AN and other eating disorders, they do not fully account for the structural, biological, and behavioral elements of the disorder. A micronutrient deficiency hypothesis offers a much more robust and comprehensive explanation at the root of these manifestations. Micronutrients are at the foundation of all physiological processes that support the growth, function, maintenance, and protection of the human body. Furthermore, they are key regulators of neurotransmitters and hormones that direct eating behaviors and emotional processing (Chafetz, 1984). The micronutrient zinc is present in every tissue and is a factor in essentially every biological process, strongly pointing to its indispensability in maintaining optimal physical and mental health.
Starvation and Malnutrition
Maladaptive eating behaviors associated with Anorexia Nervosa are strongly linked to structural, neurological, and biochemical deficits. Although food avoidance and fear of weight gain among AN patients have social and environmental influences, research data provide many biological explanations for the interaction of its cognitive, affective, and appetitive components. A greater prevalence of eating disorders in Westernized cultures suggests that inherent mechanisms predispose some individuals to fear and anxiety that can drive restrictive, irrational actions (Strober, 2004). Further research also highlights a neurobiological link between childhood temperaments characterized by anxiety, obsessions, and perfectionism and the development of AN (Kaye et al., 2013).
Functional magnetic resonance imaging (fMRI) studies demonstrate specific patterns in areas of the brain of individuals with AN, reflecting mechanisms that drive abnormal choices and persistent behaviors. At least eight human studies support a direct relationship between differences in grey and white matter volumes shown in AN patients and alterations in function and behavior (King et al., 2018). Individuals with Anorexia Nervosa consistently show dysfunctions in dorsal and striatal circuits and the cerebral cortex that disrupt dopamine and serotonin pathways and can be traced to altered interoception and distortions of taste and appetite (Kaye et al., 2009; Kaye et al., 2013). Structural abnormalities also point to aberrant reward responses impairing the ability of individuals with AN to experience reward and pleasure. A connection between these impairments and improper fear conditioning compel choices towards pathological deprivation (Keating et al., 2012). While it is not entirely clear whether brain irregularities precede or follow malnutrition or the degree to which comorbid conditions or medications may influence these observations, structural evidence supports adaptive changes in response to extreme food intakes that interfere with eating behaviors.
Low protein consumption is of distinct importance in Anorexia Nervosa, particularly considering inadequate intake of the amino acid tryptophan, a dietary precursor to the neurotransmitter serotonin. Current and recovered AN patients depleted of tryptophan demonstrate significant reductions in the Tryptophan-to-Long, Neutral Amino Acid ratio, indicating its rapid utilization following deprivation, and significant decreases in self-reported anxiety (Kaye et al., 2003). Starvation resulting in insufficient tryptophan upregulates the brain’s sensitivity to serotonin, with implications for mood and behavior. Serotonin hypersensitivity induces a response to protein consumption that increases anxiety and irritability, perpetuating the avoidance of food and resistance to change (Arnold, 2016).
While the devastating effects of malnutrition in Anorexia Nervosa are multifactorial, zinc deficiency may be one of the earliest predictors. Beyond its fundamental importance for all of human health, zinc is essential for optimal brain function. The mechanistic roles of zinc in enzyme structure and function, synaptic transmission, hormone regulation, and fatty acid metabolism provide adequate biological evidence to explore the role of zinc deficiency in AN (Bhatnagar et al., 2001). In addition to AN, many neurological disorders are linked to zinc deficiency, including autism spectrum disorders, ADHD, depression, and schizophrenia. Both animal and human studies associate zinc deprivation with impairments in learning, memory, attention, motor activity, and emotional regulation, all with implications for eating disorder symptoms and behaviors (Hagmeyer et al., 2015).
Zinc Deficiency
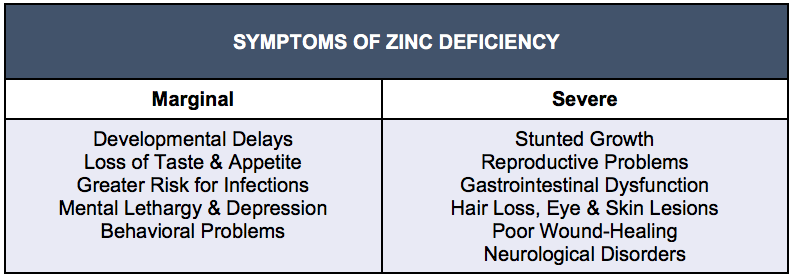
Globally, risk for zinc deficiency is estimated at a mean of 17%, with rates ranging from 7.5% in higher-income countries to 30% in Southeast Asian regions and over 50% in some developing countries (Hagmeyer et al., 2015; Jurowski et al., 2014). Designated as a growing public health problem, zinc deficiency is tied to a characteristic pattern of symptoms including poor physical and sexual development, sensory impairments, and loss of appetite, lethargy, depression, and irritability. While these representative signs and symptoms can manifest within days in moderate to severe zinc depletion, a mild-to-moderate deficiency may have subtler, undetected effects with potentially irreversible damage (Hagmeyer et al., 2015; Prasad, 2013). Marginally adequate zinc may not produce visible signs but still be suboptimal for brain function and long-term maintenance of mental stability (Arnold & Disilvestro, 2005).
Intestinal absorption of zinc is estimated to be only 33%, with significant variation based on dietary composition and tissue status. Though not stored in any tissue and in constant flux, zinc is primarily found within cells and their organelles confirming its importance for DNA and RNA structure and gene expression. Serum zinc represents only 0.1% of whole body supply due to rapid tissue demands. Homeostatic levels of zinc in the brain and other tissues are tightly controlled by tissue-specific transporters, and data show the body’s ability to maintain necessary supplies over a 10-fold variation in intake (Roohani et al., 2013).
Individual requirements for zinc can differ widely by geographical location and culture based on variations in climate, stress levels, rates of infection, and dietary composition. Societies with high dietary intakes of cereal grains and legumes and with low consumption of animal protein are at highest risk of deficiency (Jurowski et al., 2014; Wapnir, 2000). The protein content of food is consistently correlated with its zinc composition. Current zinc status directs dynamic rates of absorption or excretion that optimize bioavailability; low dietary zinc upregulates absorption and minimizes excretion in order to adapt to higher needs. Several randomized controlled trials have produced evidence that individuals consuming a low-zinc diet may have significantly greater absorptive capacity. A zinc-deficient state can also vary relative to metabolic requirements. Homeostatic responses to weight reduction may decrease immediate demands, yet weight repletion requires higher amounts; indeed, malnourished children require 15 to 20 times the typical requirement as a result of depletion and impaired absorption (Casper et al., 1980; Roohani et al., 2013).
The highest concentration of zinc is found in the hippocampus and cerebral cortex, where it resides in neural synaptic vesicles. As a modulator of synaptic transmission, zinc essentially functions as a neurotransmitter. The quantity of zinc maintained in brain tissue underlies its impact on mental function and behavior. Unfortunately, zinc status is difficult to assess and monitor, with dynamic fluctuations of up to 20% throughout a 24-hour period in response to intake. Normal serum zinc levels are often reported with severe zinc deficiency; therefore, clinical evaluations of zinc status often measure serum, plasma, hair, and urinary levels. Taste function and response to zinc therapy are considered the best-available biomarkers (Bhatnagar & Taneja, 2001; Casper et al., 1980).
Zinc deficiency was not a consideration in human disease until 1961, when a 21-year-old Iranian male with a diet containing only flatbread, potatoes, and milk presented with anemia and severe stunting of growth and sexual maturation. His remarkable response to zinc therapy encouraged research into the clinical and public health implications of this overlooked nutrient. Zinc deficiency is associated with multiple physiological impairments affecting the skin, gastrointestinal tract, central nervous system, immune system, bones, and reproductive system. Poor growth and development, diarrhea, and infection are commonly observed in populations where dietary zinc is inadequate. As a signaling molecule for immune cells, zinc regulates cell differentiation and cytokine production, with anti-inflammatory effects (Prasad, 2013; Wapnir, 2000).
Table 1: Various Manifestations by Degree of Zinc Deficiency
Zinc is also an essential factor in nutrient metabolism through its participation in the structure, storage, and activity of insulin (Brandao-Neto et al., 1995). The requirement for zinc in insulin signaling for pancreatic function and blood glucose regulation has significant implications for appetite, digestion, and eating behavior. Appetite suppression is a paramount symptom of zinc deficiency, strongly suggesting a critical role for zinc in the regulation of food intake. Experimental evidence reveals that anorexia and consequent weight loss following zinc deficiency follow repeating patterns that are independent of tissue concentrations (Humphries et al., 1989). Zinc may also mediate food intake and appetite by influencing the expression of hypothalamic neuropeptides such as Neuropeptide Y (NPY). A reduction in NPY released from the hypothalamus inhibits normal feeding behavior by inducing “NPY-resistance” and lowering its orexigenic effects (Levenson, 2003). Furthermore, clinical data showing the exclusive response of appetitive hormones to oral zinc indicate the involvement of zinc receptors in the activity of the brain-gut axis. NPY is also regulated by leptin, a neuropeptide that is involved in body weight management and is known to cross the blood-brain barrier. Leptin function is disrupted by zinc deficiency, reducing body fat. The discovery of leptin receptors in reproductive tissue provides an explanatory mechanism for the link between zinc deficiency and amenorrhea (Shay & Mangian, 2000).
The necessity for zinc in the metabolism of other micronutrients as well as fatty acids has more direct consequences for the brain and central nervous system. The activation of Vitamin B-6 to Pyridoxal-5-Phosphate (P5P), a key component in at least 4% of all enzymatic reactions, requires zinc. Zinc is also needed for the delta-6-desaturase enzyme that represents a critical step in the metabolism of essential fatty acids, with implications for the integrity of neural membranes. Secondary transformation of these lipids into neurotransmitters, prostaglandins, and other neurochemicals suggest a network of dysfunctional effects as a result of zinc deficiency. Zinc is intricately involved in the synthesis, regulation, and/or receptor function of serotonin, melatonin, dopamine, GABA, and glutamate. Detrimental impacts on cellular integrity, energy metabolism, antioxidant processes, and hormone synthesis deliver indirect cognitive, behavioral, and sensory effects on the brain (Arnold & Disilvestro, 2005).
Zinc and Anorexia Nervosa
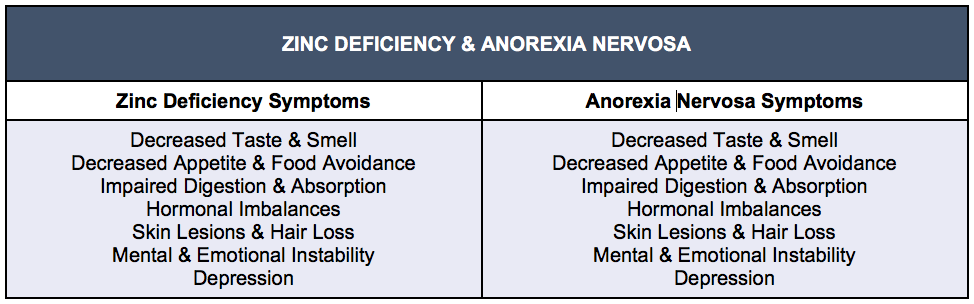
Robust clinical and epidemiological research points to a bi-directional relationship between zinc deficiency and Anorexia Nervosa, suggesting that more than half of AN patients are zinc deficient (Humphries et al., 1989). Long-term medical and hormonal consequences of AN may be induced or exacerbated by depletion of zinc either through dietary restriction, purging behaviors, or consumption of foods low in zinc. Symptoms of zinc deficiency, whether inherent or acquired, include rough skin, hair loss, mood instability, and appetite suppression, and are almost indistinguishable with many of the symptoms of Anorexia Nervosa (Van Vorhees & Riba, 1992). Multiple biomarkers of zinc status from studies of AN patients reflect consistently low levels. A selected study by Katz, et al., reported zinc intakes in 15 adolescents with AN that were significantly lower than the RDA that were reflected in reductions in urinary zinc as well as taste acuity. Most remarkably, more than 70% of these patients had amenorrhea and 66% presented with skin abnormalities. The degree of weight loss predicted almost 50% of the variation in urinary zinc and was also predictive of comorbid depression (Katz et al., 1987).
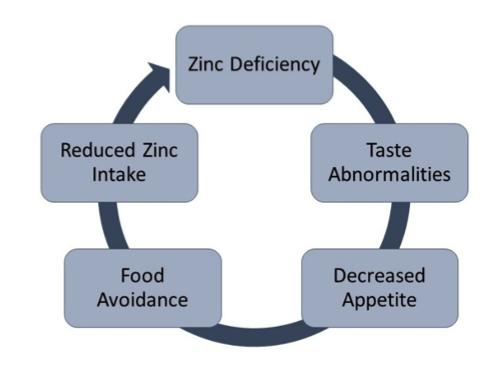
Altered sensory mechanisms may represent the most direct explanations for the relationship between zinc and food avoidance in Anorexia Nervosa. As an essential factor in the enzymatic processing of taste and smell, zinc deficiency is often first detected by loss of these senses. Depletion of zinc in both humans and animals induces food avoidance within three to five days, much earlier than any other signs and symptoms of deficiency occur (Shay & Mangian, 2000). Rapid changes in appetite following zinc depletion indicate that calorie restriction is an effect rather than a cause of food avoidance. Food restriction and malnutrition significantly affect intestinal absorption. Postprandial data suggest that AN patients may also have a reduced ability to absorb zinc due to impairments in zinc-dependent metalloenzymes (Dinsmore et al., 1985). Furthermore, an overall catabolic status associated with AN directs greater tissue uptake and retention of zinc, resulting in lower bioavailability (Ainley et al., 1986). Zinc malabsorption and suboptimal bioavailability for the brain influence mood and appetite, reducing the desire to eat and perpetuating the cycle of deficiency.
Figure 1: The Vicious Cycle of Zinc Deficiency
Micronutrient deficiencies are rarely examined and are underestimated and under-acknowledged in eating disorders despite the clear link between starvation and malnutrition. Prolonged starvation in AN blunts metabolic rates, triggers lean muscle catabolism, and has both immediate and long-term effects on zinc (Tannhauser et al., 2001). The substantial roles of zinc in the human body encompass three major categories: structural, regulatory, and catalytic. Most notably, zinc is directly or indirectly involved in over 300 enzyme reactions in the body that build, protect, and repair tissues, direct metabolic processes, and regulate neural activities involved with sensation, mood, and behaviour (Brandao-Neto et al., 1995). Strong experimental evidence from animal studies has led to the induction of zinc deficiency as a reliable model of anorexia, depression, and anhedonia.
Vegetarian diets are common among AN patients, leading many to consider it a major risk factor. Meat avoidance is rising among adolescent females, potentially explaining concurrent declines in zinc levels in this population. A sample of 45 Israeli teenagers with AN reported rates of meat avoidance 6.5 times that of their healthy peers (Tannhauser et al., 2001). Overall, vegetarians consume fewer calories, have lower body weights, and are more physically active than non-vegetarians. Vegetarian females with AN are often victims of the “female athlete triad”, associated with inadequate energy intakes to meet physical demands, resulting in menstrual dysfunction and reduced bone density. Studies suggest that vegetarian patients with Anorexia Nervosa have lower initial weights and longer illness duration despite non-significant differences in energy intake (Bakan et al., 1993).
In addition to suboptimal protein, fat, and calcium, vegetarians with AN reflect substantially reduced zinc status in comparison to non-vegetarian patients. Fiber consumption in vegetarians is estimated at two to four times average intakes, directly related to zinc status. Red meat consumption and phytate-to-zinc ratios show opposing correlations with serum zinc (Freeland-Graves et al., 1980; Gibson et al., 2001). Clinical data suggests that low protein consumption and meat avoidance may be a consequence of zinc deficiency through its influence on taste perception and amino acid metabolism. Early diets that eschew animal protein are likely contributors to long-term persistence of zinc deficiencies that may predispose individuals to abnormal eating patterns and eating disorders later in life. The etiology of AN is certainly a function of this circular cycle of zinc deficiency contributing to an altered taste and appetite that influences food avoidance, resulting in malnutrition and insufficient zinc intake.
Nutrition is a critical factor during pubertal development in cooperation with other biological, physical, and psychological processes, and is estimated to contribute to at least 25% of its variability in timing of onset. Changes in nutritional and metabolic demands during puberty increase requirements for energy, protein, iron, calcium, folate, and zinc. Calorie and protein malnutrition and low body weight during reproductive maturation may delay or halt the onset or progress of puberty in both males and females. Extreme dieting and exercise behaviors in adolescents with AN have drastic influences on the susceptibility and course of the disorder. Anorexia Nervosa is linked to dysfunctions in the secretion of growth, reproductive, and appetitive hormones, including IGF-1, leptin, luteinizing hormone, follicle-stimulating hormone, and gonadotropin-releasing hormone (Soliman et al., 2014).
Heritability of Anorexia Nervosa is estimated at 50 to 75%, with a relative risk of at least 11% in females having a first-degree relative with a history of AN (Thornton et al., 2017). Genetic variation in at least two of the 22 known zinc transporters are linked to specific inherited zinc deficient conditions. Unknown genetic variants likely underlie more widespread deficiency states and disease phenotypes. Since at least 10% of proteins in the body can bind zinc, a multitude of inherited mutations are likely to predict risk and severity of zinc related disorders such as AN (Kambe et al., 2015). In a fascinating twin study by Klump, et al., it was revealed that genetic influences on eating disorder risk differ by stage of development. Longitudinal data collected from almost 400 pairs of female twins indicated that genetic pressures exert their greatest effects during puberty, with particular implications for ovarian hormones. In combination with environmental factors, genetic risk is a significant contributor to the etiology of AN (Klump et al., 2008).
Genetic variation in the expression of Brain-Derived Neurotrophic Factor (BDNF) offers another etiological link between zinc deficiency and AN. BDNF is a key factor in neurogenesis and neural integrity, synaptic plasticity, and cognitive function, and its activity is significantly related to zinc status (Travaglia & La Mendola, 2017). Reduced levels of BDNF in specific regions of the brain are associated with many neurodegenerative disorders including depression. Evidence that both zinc deficiency and chronic stress deplete BDNF levels was demonstrated by Cieślik, et al., showing significant up-regulation of BDNF production in response to zinc therapy (Cieśelik et al., 2011).
High levels of stress, elevated estrogen, excessive dieting and exercise behaviors during adolescence, particularly in females, negatively impact zinc status and are associated with a higher risk of developing an eating disorder. Females between 12 and 25 appear to be at highest risk, with AN often manifesting in combination with physiologically and psychologically stressful events including puberty, college, and marriage. Zinc-deficient adolescents with AN are also at much greater risk for depression and anxiety through the impacts of metabolic impairments in limbic regions of the brain where zinc is concentrated (Katz et al., 1987). This age group is also most likely to be affected by the depletive effects on zinc status by oral contraceptives and other hormonal dynamics. Elevated estrogen by contraceptives has been shown to deplete zinc, increasing risk in an already vulnerable population (Gibson et al., 2001).
The ubiquitous relationship between Anorexia Nervosa and depression is mediated through multiple zinc-related mechanisms including insufficient enzyme synthesis and function, neural and hormonal disruption, and overactivation of the HPA axis and the immune system. Symptoms of mild to severe depression are significantly correlated with zinc status (Maes et al., 1994). Recent evidence in a study by Ren, et al., also demonstrated the role of zinc in catecholamine regulation. Advanced laboratory methods have allowed researchers to discover that zinc optimizes synaptic neurotransmitter release by controlling the timing of the exocytosis process. By slowing the rate of pore formation in exocytotic vesicles, zinc increases the efficiency of neurotransmission, increasing synaptic strength and plasticity. These novel findings further implicate zinc’s involvement in neurochemical modulation that is lacking in zinc-deficiency and eating disorders (Ren et al., 2017).
Zinc has antidepressant effects by enhancing Brain-Derived Neurotrophic Factor (BDNF) and through its inhibitory action on N-methyl-d-aspartate (NMDA) glutamate receptors (Nowaket al., 2005). NMDA receptors are most sensitive to zinc and magnesium, two micronutrients linked to depression and suicide. Hippocampal brain tissue of 17 suicide victims revealed 30 to 40 percent lower inhibition of NMDA receptors by zinc and magnesium, indicating aberrations in glutamate regulation (Sowa-Kućma et al., 2013). Overexcitation of glutamatergic neurons is associated with neurotoxicity and oxidative stress in areas of the brain related to emotion and cognition.
Table 2: Indistinguishable Symptoms of Zinc Deficiency and Anorexia Nervosa
Supplementation for Anorexia Nervosa
Due to inadequate understanding and comprehension of AN disease pathology and zinc’s variety of mechanistic influences, zinc supplements are not commonly used treatments. While not every case of Anorexia Nervosa can be linked to zinc deficiency, a wealth of convincing evidence supports zinc supplements for prevention and treatment, particularly in high-risk individuals. Consistent data linking zinc deficiency with the physiological and psychological symptoms of AN strongly promote early clinical assessments of zinc status in all AN patients. As zinc requirements are highly individual and variable by age, developmental stage, degree of weight loss, disease duration, and presence of comorbid conditions, thorough and accurate testing is critical.
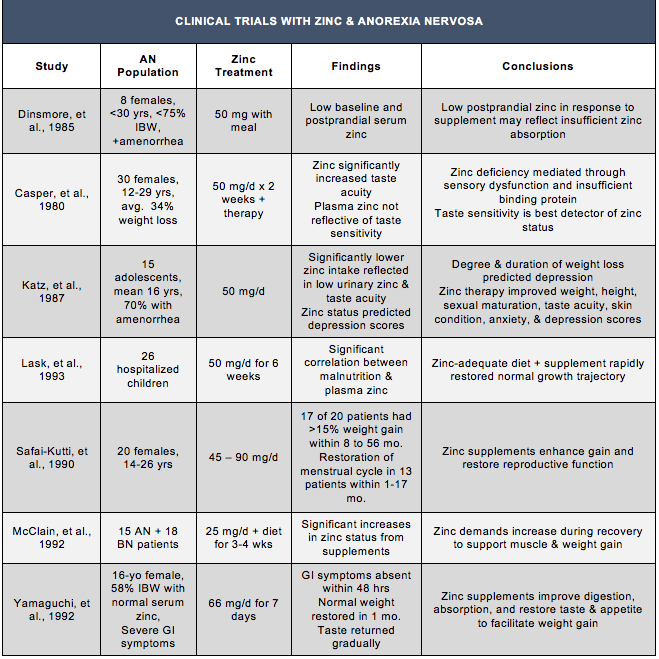
Supplementation trials with zinc began in the 1970s, leading to the establishment of the Recommended Daily Allowance in 1974. In 1983, Smith and Simpson provided the first clinical demonstration of successful treatment with zinc for Anorexia Nervosa in a 13-year-old female. Diagnosed by a zinc taste test and supplemented with 45 mg of liquid zinc per day, the patient’s mood, appetite, and weight gain improved rapidly and a normal weight trajectory was restored within 10 months. Further evidence of zinc’s efficacy was revealed when a period of heightened stress resulted in a return of zinc deficiency, weight loss, and depressed mood that was corrected with returning the patient to a therapeutic dose of zinc (Schauss & Costin, 1989). Research suggests that zinc supplements augment weight gain and recovery by improving digestion, nutrient absorption, and restoring sensory function to increase taste and appetite. Restoration of taste acuity by zinc therapy is often a critical first step in regulating appetite and normal eating behaviors.
Adjunctive treatments with zinc for AN patients have also been shown to enhance BMI restoration and balance neurotransmitter levels. Correcting neurotransmitter abnormalities with zinc therapy also produces clinically significant improvements in mood that facilitate recovery. Multiple studies recognizing the symptom similarities between zinc deficiency and Anorexia Nervosa show a positive correlation between supplementation with zinc and improved rates of recovery through weight restoration and mood stabilization. Therapeutic doses of oral zinc supplements have produced profound responses in supporting weight gain during AN recovery. A double-blind crossover study in 26 zinc-deficient children with AN given 50 mg per day of zinc in addition to a nutritious diet for six weeks resulted in rapid restoration of a normal growth trajectory in addition to stabilizing plasma zinc (Lask et al., 1993). In a separate study, doses between 45 and 90 mg in 20 young females with Anorexia Nervosa resulted in rapid weight restoration in 85% of patients. No patients receiving zinc therapy continued to lose weight, and 13 of the 20 women recovered their menstrual cycle within 1 to 17 months (Safai-Kutti, 1990).
The majority of clinical trial data support a preventive or maintenance dose of zinc of 15 mg per day, supplied from both food and supplements, and at least 2 months with 15 to 20 mg for treatment of patients with a known zinc deficiency (Silveria et al., 2013). The form of supplemental zinc appears to be a critical consideration, especially when used for a therapeutic purpose. Many supplemental forms of zinc employ inorganic zinc oxide or zinc sulfate, which have limited bioavailability. Chelated forms of zinc such as zinc orotate, zinc citrate, or zinc gluconate, are more readily absorbed, and data suggests that zinc orotate results in the greatest tissue uptake (Corriher, 2010). While the majority of clinical trials with moderate doses of zinc report no adverse effects or incidents of toxicity, some studies using higher doses have indicated concerns with using zinc supplements containing 50 to 150 mg or more. These moderate doses have been linked to gastrointestinal problems and headache, and high doses can be toxic, with 300 mg inducing significant immune suppression (Arnold & Disilvestro, 2005). Long-term supplementation with higher doses of zinc is also linked to reduced copper status and alterations in iron metabolism through disruptions in metallothionein activity (NIH, 2016)
Recovering AN patients should also focus on including dietary sources of zinc in addition to oral zinc supplements. In order to facilitate weight gain and anabolic growth, zinc demands are especially high during the healing process, and ongoing zinc deficiencies may continue to sustain the chronicity and severity of the disorder. Food sources rich in zinc, including red meat, poultry, seafood, and eggs, are recommended for optimal absorption and bioavailability. Fortified food products can also provide beneficial amounts (NIH, 2016). However, zinc-adequate diets may not be enough to meet the elevated needs during recovery from AN. A supplementation trial by McClain, et al., in 33 hospitalized eating disorder patients demonstrated that 25 mg per day of zinc acetate in addition to a zinc-adequate diet was necessary to restore zinc status; patients consuming only dietary zinc remained deficient (McClain et al., 1992).
Although there is substantial research to support the inclusion of zinc supplements in the treatment of Anorexia Nervosa, zinc deficiency represents only one factor in the etiology and pathophysiology of this complex disorder. Restoration of zinc status represents a distinct aspect of a multi-component intervention for AN patients; however, an overall malnourished state requires comprehensive dietary adequacy, additional concentrated micronutrients, essential fatty acid support, and provisions for the intestinal microbiome (Kleiman et al., 2016; Yehuda & Rabinovitz, 2016). As a particularly persistent disorder, Anorexia Nervosa necessitates laboratory and psychological monitoring to provide the optimal treatment for sustained recovery. The contribution of zinc deficiency to the origin, course, and outcome of Anorexia Nervosa cannot be overestimated.
Table 3: Clinical Trial Results for Zinc Therapies in Anorexia Nervosa Patients
Startling international cases of euthanasia and assisted suicide in patients abandoning any hope of treatment serve as testimonies to the tragic failure of the medical community to help or heal those with Anorexia Nervosa. In 2014, a 29-year-old New Jersey woman with severe AN won the legal right to refuse further treatment efforts, choosing instead to starve to death after 16 years of futile force-feedings and other conventional interventions (Forster, 2016). Exhaustion with ineffective therapies was also reflected in at least two heartbreaking cases of young women in Belgium utilizing assisted-suicide services after suffering through rounds of unsuccessful treatments (Buchanan, 2015; Fiano, 2013). Finally, following at least 15 years of resistance to intensive treatments in a young woman overcome with AN, physical disabilities, and depression, doctors in Holland abandoned their efforts and allowed her to end her own life (Bilger, 2016).
Surely this is only a sampling of such cases, imploring a more urgent need for clinicians to rethink our current treatment models. Unbelievably, a recent panel of mental health experts in the US gathered to consider end-of-life care for AN patients as a “supportive and compassionate” practice. It is ethically imperative that those of us charged with “doing no harm” hold the greatest dedication to achieving successful outcomes for patients and encourage the belief that life is worth living. While conventional methods continue to fail and patients suffer and die from the destructive effects of AN, clinicians must not ignore the effects of malnutrition and the undeniable role that zinc deficiency plays in Anorexia Nervosa.
Competing Interests
Dr. Greenblatt receives royalties from the self-published book Answers to Anorexia, CreateSpace2018 and is a consultant for Pure Encapsulations, a nutritional supplement company.
Desiree Delane has no competing interests.
References
Abbate-Daga, G., Amianto, F., Delsedime, N., De-Bacco, C., & Fassino, S. (2013). Resistance to treatment and change in anorexia nervosa [corrected]: a clinical overview. BMC Psychiatry, 13, 294. http://doi.org/10.1186/1471-244X-13-294
Ainley, C., Cason, J., Carlsson, L., & Thompson, R. (1986). Zinc state in anorexia nervosa. British Medical Journal, 293(October), 992–993.
Amani, R., Saeidi, S., Nazari, Z., & Nematpour, S. (2010). Correlation between dietary zinc intakes and its serum levels with depression scales in young female students. Biological Trace Element Research, 137(2), 150–158. http://doi.org/10.1007/s12011-009-8572-x
Arnold, C. (2016). The Challenge of Treating Anorexia in Adults. Retrieved February 1, 2018, from https://www.theatlantic.com/health/archive/2016/03/treating-anorexia-in-adults/475845/
Arnold, L. E., & Disilvestro, R. A. (2005). Zinc in Attention-Deficit / Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology, 15(4), 619–627.
Bakan, R., Birmingham, C. L., Aeberhardt, L., & Coldner, E. M. (1993). Dietary zinc intake of vegetarian and nonvegetarian patients with anorexia nervosa. International Journal of Eating Disorders, 13(2), 229–233. http://doi.org/10.1002/1098-108X(199303)13:2<229::AID-EAT2260130211>3.0.CO;2-1
Beumont, P., Beumont, R., Hay, P., Beumont, D., Birmingham, L., Derham, H., … Weigall, S. (2005). Australian and New Zealand Clinical Practice Guidelines for the Treatment of Anorexia Nervosa. The Journal of Life Long Learning in Psychiatry, 3(4). Retrieved from http://ps.psychiatryonline.org/data/Journals/FOCUS/2626/618.pdf
Bhatnagar, S., & Taneja, S. (2001). Zinc and cognitive development. British Journal of Nutrition, 85(S2), S139. http://doi.org/10.1079/BJN2000306
Bilger, M. (2016). Woman Dealing With Depression , Anorexia Euthanized When Doctors Decide She Can ’ t be Cured. Retrieved February 12, 2018, from http://www.lifenews.com/2016/05/11/woman-dealing-with-depression-anorexia-euthanized-when-doctors-decide-she-cant-be-cured/
Brandao-Neto, J., Stefan, V., Mendonca, B., Bloise, W., & Castro, A. (1995). The Essential Role of Zinc in Growth. Nutrition Research, 15(3), 335–358.
Buchanan, R. (2015). Right to die: Belgian doctors rule depressed 24-year-old woman has right to end her life. Retrieved February 12, 2018, from http://www.independent.co.uk/news/people/right-to-die-belgian-doctors-rule-depressed-24-year-old-woman-has-right-to-end-her-life-10361492.html
Casper, R., Kirschner, B., Sandstead, H., Jacob, R., & Davis, J. (1980). An evaluation taste function of trace metals , vitamins , in anorexia nervosa. Am J Clin Nutr, 33, 1801–1808.
Chafetz, M. (1984). Anorexia: A Micronutrient Model. The Southern Psychologist, 2(1), 39–47.
Cieśelik, K., Sowa-Kućma, M., Ossowska, G., Legutko, B., Wolak, M., Opoka, W., & Nowak, G. (2011). Chronic unpredictable stress-induced reduction in the hippocampal brain-derived neurotrophic factor (BDNF) gene expression is antagonized by zinc treatment. Pharmacological Reports, 63(2), 537–543.
Corriher, S. (2010). Not All Zinc Supplements Are Created Equal! Retrieved February 6, 2018, from https://healthwyze.org/reports/338-why-zinc-should-be-taken-daily-and
Dinsmore, W., Alderdice, J., McMaster, D., Adams, C., & Love, A. (1985). Zinc Absorption in Anorexia Nervosa. The Lancet, (May), 1041–1042.
Eating Disorders Coalition, E. (2016). Facts About Eating Disorders: What the Research Shows. EDC: Eating Disorders Coalition. Retrieved from http://eatingdisorderscoalition.org.s208556.gridserver.com/couch/uploads/file/fact-sheet_2016.pdf
Fazeli, P. K., Calder, G. L., Miller, K. K., Misra, M., Lawson, E. A., Meenaghan, E., … Klibanski, A. (2012). Psychotropic medication use in anorexia nervosa between 1997 and 2009. International Journal of Eating Disorders, 45(8), 970–976. http://doi.org/10.1002/eat.22037
Fiano, C. (2013). Belgian woman suffering from anorexia euthanized. Retrieved February 12, 2018, from https://www.liveaction.org/news/belgian-woman-suffering-from-anorexia-euthanized/
Forster, K. (2016). Woman with Severe Anorexia Has Right to Starve to Death, US Court Finds. Retrieved February 12, 2018, from http://www.independent.co.uk/life-style/health-and-families/health-news/anorexia-right-to-starve-death-die-eating-disorder-force-feeding-compulsory-treatment-beat-new-a7433381.html
Frank, G. K., Shott, M. E., Hagman, J. O., & Mittal, V. A. (2013). Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. The American Journal of Psychiatry, 170(10), 1152–60. http://doi.org/10.1176/appi.ajp.2013.12101294
Frank, G. K. W. (2014). Advances from neuroimaging studies in eating disorders. CNS Spectrums, 20(4), 391–400. http://doi.org/10.1017/S1092852915000012
Freeland-Graves, J. H., Bodzy, P. W., & Eppright, M. A. (1980). Zinc status of vegetarians. Journal of the American Dietetic Association, 77(6), 655–661.
Garner, D. M., Anderson, M. L., Keiper, C. D., Whynott, R., & Parker, L. (2016). Psychotropic medications in adult and adolescent eating disorders: clinical practice versus evidence-based recommendations. Eating and Weight Disorders, 21(3), 395–402. http://doi.org/10.1007/s40519-016-0253-0
Gibson, R. S., Heath, A.-L. M., Limbaga, M. L. S., Prosser, N., & Skeaff, C. M. (2001). Are changes in food consumption patterns associated with lower biochemical zinc status among women from Dunedin, New Zealand? British Journal of Nutrition, 86(1), 71. http://doi.org/10.1079/BJN2001370
Hagmeyer, S., Haderspeck, J. C., & Grabrucker, A. M. (2015). Behavioral impairments in animal models for zinc deficiency. Frontiers in Behavioral Neuroscience, 8(January), 1–16. http://doi.org/10.3389/fnbeh.2014.00443
Humphries, L., Vivian, B., Stuart, M., & McClain, C. J. (1989). Zinc deficiency and eating disorders. The Journal of Clinical Psychiatry, 50(12), 456–459.
Jurowski, K., Szewczyk, B., Nowak, G., & Piekoszewski, W. (2014). Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. Journal of Biological Inorganic Chemistry, 19(7), 1069–1079. http://doi.org/10.1007/s00775-014-1139-0
Kambe, T., Fukue, K., Ishida, R., & Miyazaki, S. (2015). Overview of Inherited Zinc Deficiency in Infants and Children. Journal of Nutritional Science and Vitaminology, 61(Supplement), S44–S46. http://doi.org/10.3177/jnsv.61.S44
Katz, R., Keen, C., Litt, I., Hurley, L., Kellams-Harrison, K., & Glader, L. (1987). Zinc Deficiency In Anorexi Nervosa. Journal of Adolescent Health Care, 8, 400–406.
Kaye, W. H., Barbarich, N. C., Putnam, K., Gendall, K. A., Fernstrom, J., Fernstrom, M., … Kishore, A. (2003). Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. International Journal of Eating Disorders, 33(3), 257–267. http://doi.org/10.1002/eat.10135
Kaye, W. H., Fudge, J. L., & Paulus, M. (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience, 10(8), 573–584. http://doi.org/10.1038/nrn2682
Kaye, W., Wierenga, C., Bailer, U., Simmons, A., & Bischoff-Grethe, A. (2013). Nothing Tastes as Good as Skinny Feels. Trends in Neuroscience, 36(2), 110–120.
Keating, C., Tilbrook, A. J., Rossell, S. L., Enticott, P. G., & Fitzgerald, P. B. (2012). Reward processing in anorexia nervosa. Neuropsychologia, 50(5), 567–575. http://doi.org/10.1016/j.neuropsychologia.2012.01.036
King, J. A., Frank, G. K. W., Thompson, P. M., & Ehrlich, S. (2018). Structural Neuroimaging of Anorexia Nervosa: Future Directions in the Quest for Mechanisms Underlying Dynamic Alterations. Biological Psychiatry, 83(3), 224–234. http://doi.org/10.1016/j.biopsych.2017.08.011
Kleiman, S., Watson, H., Bulik-Sullivan, E., Huh, E., Tarantino, L., Bulik, C., & Carroll, I. (2016). The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom Med, 77(9), 969–981. http://doi.org/10.1097/PSY.0000000000000247.The
Klump, K. L., Burt, S. A., Mcgue, M., & Iacono, W. G. (2008). Changes in Genetic and Environmental Influences on Disordered Eating Across Adolescence. American Medical Association, 64(12), 1409–1415.
Lask, B., Fosson, A., Rolfe, U., & Thomas, S. (1993). Zinc deficiency and childhood-onset anorexia nervosa. Journal of Clinical Psychiatry, 54(2), 63–66.
Levenson, C. W. (2003). Zinc regulation of food intake: New insights on the role of neuropeptide Y. Nutrition Reviews, 61(7), 247–249. http://doi.org/10.1301/nr.2003.jul.247-249
Maes, M., D’Haese, P. C., Scharpé, S., D’Hondt, P., Cosyns, P., & De Broe, M. E. (1994). Hypozincemia in depression. Journal of Affective Disorders, 31(2), 135–140. http://doi.org/10.1016/0165-0327(94)90117-1
McClain, CJ, Stuart, MA, Vivian, B, McClain, M, Talwalker, R, Snelling, L, Humphries, L. (1992). Zinc status before and after zinc supplementation of eating disorder patients. Journal of the American College of Nutrition, 11(6), 694–700.
Nowak, G., Szewczyk, B., & Pilc, A. (2005). Zinc and depression. An update. Pharmacological Reports, 57(6), 713–718. http://doi.org/10.1038/nprot.2006.224
Prasad, A. S. (2013). Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Advances in Nutrition, 4, 176–190. http://doi.org/10.3945/an.112.003210.176
Ren, L., Pour, M. D., Majdi, S., Li, X., Malmberg, P., & Ewing, A. G. (2017). Zinc Regulates Chemical-Transmitter Storage in Nanometer Vesicles and Exocytosis Dynamics as Measured by Amperometry. Angewandte Chemie – International Edition, 56(18), 4970–4975. http://doi.org/10.1002/anie.201700095
Roohani, N., Hurrell, R., Kelishadi, R., & Schulin, R. (2013). Zinc and its importance for human health: An integrative review. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences, 18(2), 144. http://doi.org/23914218
Safai-Kutti, S. (1990). Oral zinc supplementation in Anorexia Nervosa. Acta Psychiatrica Scandinavica, 82, 14–17.
Schauss, A., & Costin, C. (1989). Zinc and Eating Disorders. New Canaan, CT: Keats Publishing, Inc.
Shay, N., & Mangian, H. (2000). Zinc and Health : Current Status and Future Directions. The Journal of Nutrition, 130, 1493S–1499S.
Silveria, L., Silvia, B., de Oliveira Ribeiro, B., Maciel, K., Favero, L., Marchini, J., & Buzzini, R. (2013). Zinc supplementation in the treatment of anorexia nervosa. Rev Assoc Med Bras, 59(4), 321–324.
Soliman, A., Sanctis, V., & Elalaily, R. (2014). Nutrition and pubertal development. Indian Journal of Endocrinology and Metabolism, 18(7), 39. http://doi.org/10.4103/2230-8210.145073
Sowa-Kućma, M., Szewczyk, B., Sadlik, K., Piekoszewski, W., Trela, F., Opoka, W., … Nowak, G. (2013). Zinc, magnesium and NMDA receptor alterations in the hippocampus of suicide victims. Journal of Affective Disorders, 151(3), 924–931. http://doi.org/10.1016/j.jad.2013.08.009
Steinhausen, H. C. (2002). The outcome of anorexia nervosa in the 20th century. American Journal of Psychiatry, 159(8), 1284–1293. http://doi.org/10.1176/appi.ajp.159.8.1284
Strober, M. (2004). Pathologic Fear Conditioning and Anorexia Nervosa: On the Search for Novel Paradigms. International Journal of Eating Disorders, 35(4), 504–508. http://doi.org/10.1002/eat.20029
Supplements, N. O. of D. (2016). Zinc: Fact Sheet for Health Professionals. Retrieved February 6, 2018, from https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
Tannhauser, P., Latzer, Y., Rozen, G., Tamir, A., & Naveh, Y. (2001). Zinc status and meat avoidance in anorexia nervosa. Int J Adolesc Med Health, 13(4), 317–326.
Thornton, L., Munn-Chernoff, M., Baker, J., Jureus, A., Parker, R., & Henders, A. (2017). The Anorexia Nervosa Genetics Initiative: Study description and sample characteristics of the Australian and New Zealand arm. Aust N Z J Psychiatry, 51(6), 583–594.
Travaglia, A., & La Mendola, D. (2017). Zinc Interactions With Brain-Derived Neurotrophic Factor and Related Peptide Fragments. Vitamins and Hormones, 104, 29–56. http://doi.org/10.1016/bs.vh.2016.10.005
Van Vorhees, AS. Riba, M. (1992). Acquired zinc deficiency in association with anorexia nervosa. Pediatric Dermatology, 9(3), 268–271.
Wapnir, R. a. (2000). Zinc deficiency, malnutrition and the gastrointestinal tract. The Journal of Nutrition, 130(5S Suppl), 1388S–92S.
Yehuda, S., & Rabinovitz, S. (2016). The Role of Essential Fatty Acids in Anorexia Nervosa and in Obesity. Crit Rev Food Sci Nutr., 56(12), 2021–35. http://doi.org/10.1016/j.jss.2009.11.00416


