.
Background
One in two Canadians will get cancer in their lifetime and one in four of them will experience depression during their diagnosis (Canadian Cancer Society, 2020). The cause of depression during cancer is often multifactorial and may include psychological, social and immunological factors as well as inflammation related to cancer or treatments (Smith, 2015). Chronic stress is common in cancer patients, leading to increased levels of catecholamines, which are associated with higher rates of depression (Smith, 2015). Although depression in cancer patients is common, it is underdiagnosed and the results are dire (Smith, 2015). Not only does depression in cancer patients significantly reduce quality of life (QoL), it is associated with higher levels of anorexia, fatigue/sleep issues as well as increased number of days in the hospital and numbers of resources used, leading to greater health-related expenses (Smith, 2015; Vollbracht et al., 2011). Cancer patients with depression are more than two times more likely than people without cancer to commit suicide (Misono et al., 2008). Studies have associated increased depression with increased metastasis and worse survival outcomes (Anderson et al., 1994).
Nutritional deficiencies are a well-observed occurrence in cancer patients due to a variety of reasons, ranging from inadequate dietary factors to increased demands associated with cancer and chemo/radiotherapy, and have dramatic implications in disease progression with some studies citing an approximated 30% increase in mortality in malnourished cancer patients (Zürcher, 2002, as cited in Gröber et al., 2016). In a 2019 study of palliative cancer patients, authors Vollbracht et al. sought to determine nutritional status and found vitamin C deficiency (defined as serum below 4.5mg/L) in 45.2% of patients, as well as deficiencies of vitamin D3 (93.5%), B6 (48.4%), and B1 (25.8%). Although associations have been observed between deficiencies in numerous vitamins and minerals, vitamin C and B6 deficiencies have significant implications in the synthesis of the neurotransmitters serotonin, norepinephrine, and dopamine (Gropper et al., 2018, pp. 307, 362). Vitamin C is a versatile nutrient in the body which was first isolated in 1928 and has since been subject to many different studies to explore its various applications and mechanisms (Gropper et al., 2018, p. 303). Humans lack the enzyme gulonolactone oxidase, which is integral to endogenous vitamin C synthesis; as such, deficiency is common in humans when demands are not met through dietary sources or supplementation (Gropper et al., 2018, p. 304). When a patient undergoes surgery, chemotherapy, or radiation, there is an increased demand by the body for vitamin C as it plays an integral role in several different biological mechanisms. (Jonas et al., 2000, as cited in Klimant et al., 2018).
Linus Pauling was the first to use intravenous (IV) vitamin C (IVC) for cancer patients in the 1970’s and in the following 50 years a significant amount of research has been undertaken on this therapy (Fritz et al., 2014). Studies have shown that high dose IVC is safe and has beneficial cancer-related effects such as increased survival times, decreased tumour growth, increased QoL, increased efficacy of conventional treatment, decreased cancer symptoms and decreased side effects from conventional cancer treatment (Fritz et al., 2014). IVC administration results in increased serum levels 100 times higher than with oral administration (Levine et al., 2011). The resulting increase in serum vitamin C is utilized by GLUT glucose transporters, which are upregulated in tumour cells and lead to substantially higher concentrations within tumours than non-cancerous tissue (Fritz et al., 2014). This upregulation of vitamin C intake in tumour cells accounts for some of the increased metabolic demand for vitamin C in cancer patients (Fritz et al., 2014). The pro-oxidant effects of high cellular levels of vitamin C increases hydrogen peroxide, which has been demonstrated to be oncolytic while being neutral to non-cancer cells due to the presence of catalase (Belin et al., 2009; Carosio et al., 2007; Fritz et al., 2014; Vissers & Das 2018). This capacity of catalase is demonstrably reduced in cancer cells with some studies noting a two-fold difference in metabolism (Doskey et al., 2016).
Oral vitamin C differs from IVC in that the gastrointestinal (GI) tract limits its absorption (Fritz et al., 2014). The maximum serum concentration oral route vitamin C can achieve is 0.25 mM, which is about 1% the change in concentration in response to IVC (Levine et al., 2011, as cited in Fritz et al., 2014). Vickers and Cassileth (2001) completed a literature review that showed that oral vitamin C was ineffective at significantly reducing cancer-related symptoms, although they did not evaluate its effects on depression. This highlights the fact that oral and IVC have significantly different pharmacologic and clinical effects and should be considered separately. The antioxidant effects of vitamin C in non-cancerous cells is another potential mechanism of action for a possible antidepressant effect (Khanzode et al., 2003; Vollbracht et al., 2011). Khanzode et al. (2003) showed that individuals with major depression have significantly increased levels of oxidative stress markers, such as serum superoxide dismutase (SOD), and increased serum malondialdehyde (MDA) and decreased plasma vitamin C levels. Multiple studies have been conducted that show patients with depression have significantly decreased vitamin C levels (Gupta et al., 2014; Schlueter & Johnston, 2011; Vollbracht et al, 2019). Zhang et al. (2011) showed that oral vitamin C administration to acutely hospitalized patients significantly improved depression. Amr. et al (2013) showed that 1 g of oral vitamin C per day alongside fluoxetine significantly reduced depression as compared to fluoxetine with placebo.
Despite preliminary evidence and possible mechanisms, no efforts have been made to synthesize the research related to the effects of IVC on depression levels in patients with cancer. The purpose of this literature review is to identify and synthesize the studies that have assessed depression levels in patients receiving IVC treatment for cancer, identify gaps and put forward recommendations for further research.
Methods
Question: Does intravenous vitamin C (IVC) have beneficial effects for depression in cancer patients?
Search Strategy: PubMed, MedLine Complete, CINAHL Plus, Web of Science, Cochrane, and PMC databases were searched in May 2020. We used search terms such as “IVC”, “Intravenous Vitamin C”, “Vitamin C”, and “Ascorbic Acid” in conjunction with terms “Depression” and “Cancer.” We included both prospective and retrospective observational studies. Bibliographies of relevant articles were searched for additional publications not identified in the search.
Inclusion Criteria:
- Human participants with confirmed cancer (any type and stage)
- Intravenous vitamin C treatment, with or without conventional treatment and with or without additional oral vitamin C dosing
- Assessment of depression
Exclusion Criteria:
- Preclinical studies (Animal, in vitro)
- Non-English publications
- Literature reviews and opinion articles
Results
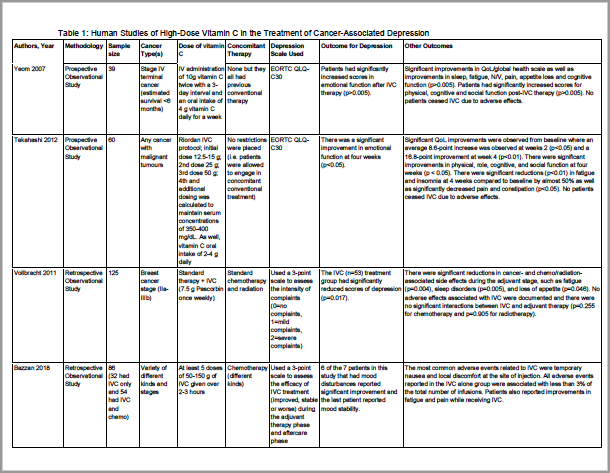
The search strategy identified 152 scholarly articles. Of these, four observational studies met criteria for inclusion. These four studies have been summarized in Table 1.
In all four studies, researchers found a benefit of IVC on depressive symptoms associated with cancer and/or cancer therapy. In the 2007 Yeom et al. study authors examined the impact of IVC and oral vitamin C supplementation on 39 patients with terminal cancer diagnoses (less than six months life expectancy) where the treatment goal was improvement of quality of life (Yeom et al., 2018). Two doses of 10 g IVC, administered within a three-day window with concurrent 4 g oral route vitamin C for one week was found to significantly improve the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) scores (Yeom et al., 2018). Analysis revealed significant improvement in all five functional scales (physical, role, emotional, cognitive, social) and significant improvement in several symptom scores such as fatigue, nausea/vomiting and appetite loss; and other symptom improvement was observed in pain and sleep disturbance (Yeom et al., 2018).
Similarly, Takahashi et. al, reported a significant improvement in EORTC QLQ-C30 scores at two weeks compared to baseline and an even more significant improvement at 4 weeks in 60 patients. This study also reported significant improvements in all five functional scales, which includes emotional function as well as fatigue, insomnia, pain and constipation (Takahashi et al., 2012).
Two retrospective studies were included in our review, Vollbracht et al. (2011) and Bazzan et al. (2018). In the Bazzan study, authors identified cases where patients had either received IVC alone, IVC+chemotherapy, or chemotherapy alone (Bazzan et al., 2018). They reported significant improvement in pain and fatigue and six out of seven patients reported improved mood (the last remained unchanged) (Bazzan et al., 2018). In the Vollbracht et al. (2011) study, authors sought to explicitly examine the impact of IVC on symptoms caused by chemotherapy and radiotherapy and foundsignificant improvements during the adjuvant therapy phase to depression as well as fatigue, sleep disorders and loss of appetite.
Table 1. Human Studies of High-Dose Vitamin C in the Treatment of Cancer-Associated Depression
Discussion
Four observational studies assessed the role of IVC for depression in cancer patients. These studies confirmed previous research in reporting improved quality of life and other cancer-related benefits (i.e. decreasing fatigue and pain) (Bazzan et al., 2018; Takahashi et al., 2012; Vollbracht et al., 2011; Yeom et al., 2007). All four studies noted a decrease of depression in cancer patients (Bazzan et al., 2018; Takahashi et al., 2012; Vollbracht et al., 2011; Yeom et al., 2007). The included studies all evaluate safety of IVC and reported no significant adverse effects associated with IVC, which is critical when considering any intervention as a proposed treatment. These findings of safety have been replicated in numerous trials and have been summarized in several meta-analyses and systematic reviews (Bazzan et al., 2018; Fritz et al., 2014; Schlueter & Johnston, 2011; Stephenson et al., 2013; Takahashi et al., 2012; Vollbracht et al., 2011; Yeom et al., 2007; Zhang et al., 2011). The consistency of these findings further supports the feasibility of future studies into IVC and cancer, including its role in the treatment of depression.
One strength of this review is that a systematic search strategy was implemented to ensure it identified all relevant studies. Another strength is that the validated questionnaire, EORTC QLQ-C30, was used in two studies, which aids in comparison of the findings (Takahashi et al., 2012; Yeom et al., 2007). One limitation of using the EORTC QLQ-C30 is that it was designed to capture overall QoL and while a subdomain assesses emotional function, it is not validated for the assessment of depression. Research does exist, however, that draws an association between EORTC scores and depression, such as a 2009 study of colorectal cancer patients that correlates those who scored over 17 points on the Beck Depression Inventory with substantially lower EORTC scores (Alacacioglu et al, 2010). Another limitation of this review is that two of the four studies were retrospective and only one study had a control group (Bazzan et al., 2018; Takahashi et al., 2012; Vollbracht et al., 2011; Yeom et al., 2007). There are several validated depression tools such as the PHQ-9, Hamilton Depression Rating Scale, and the Beck Depression Inventory. In future studies, it is important that a validated tool is used in order to adequately assess the impact treatment has on depression symptoms.
Cancer and depression share some common pathophysiologic characteristics, such as decreased immune function and altered adrenal function and vitamin C could possibly alleviate both (Cui et al., 2019; Smith, 2015). Not only can cancer cause depression, but depression can cause chronic stress, which may contribute to the development and progression of cancer (Cui et al., 2019; Moreno-Smith et al., 2010; Song et al., 2017). Chronic stress increases epinephrine, which is known to suppress the immune system and high enough levels for a long enough time can potentially contribute to carcinogenesis (Cui et al., 2019). Studies have been completed in-vitro and in-vivo that show vitamin C decreases chronic stress-induced cancer cells, indicating that vitamin C possibly plays a role in tumour growth via this mechanism (Cui et al., 2019). Vitamin C is also vital for adrenal function, which plays a crucial role in depression via the hypothalamic-pituitary-adrenal (HPA) axis, therefore it is important to have sufficient levels for emotional wellbeing (Kessing et al., 2011; Patak et al., 2004).
Conclusion
The results presented suggest that IVC could potentially have a beneficial effect on levels of depression in patients with cancer; however, more research is needed. The studies completed to date lacked an assessment tool designed to capture changes in depression severity. Prospective clinical trials using validated assessment tools and control groups are needed to further study the potential role of this therapy in the treatment of this highly prevalent and disabling condition.
The authors declare that they have no competing interests.
References
Alacacioglu, A., Binicier, O., Gungor, O., Oztop, I., Dirioz, M., & Yilmaz, U. (2010). Quality of life, anxiety, and depression in Turkish colorectal cancer patients. Support Care Cancer, 18(4), 417–421. https://doi.org/10.1007/s00520-009-0679-2
Alexander, M. S., Wilkes, J. G., Schroeder, S. R., Buettner, G. R., Wagner, B. A., Du, J., Gibson-Corley, K., O’Leary, B. R., Spitz, D. R., Buatti, J. M., Berg, D. J., Bodeker, K. L., Vollstedt, S., Brown, H. A., Allen, B. G., & Cullen, J. J. (2018). Pharmacologic ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Research, 78(24), 6838–6851. https://doi.org/10.1158/0008-5472.CAN-18-1680
Amr, M., El-Mogy, A., Shams, T., Vieira, K., & Lakhan, S. E. (2013). Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. Nutrition Journal, 12, 31. https://doi.org/10.1186/1475-2891-12-31
Andersen, B. L., Kiecolt-Glaser, J. K., & Glaser, R. (1994). A biobehavioral model of cancer stress and disease course. The American Psychologist, 49(5), 389–404.
Bazzan, A. J., Zabrecky, G., Wintering, N., Newberg, A. B., & Monti, D. A. (2018). Retrospective evaluation of clinical experience with intravenous ascorbic acid in patients with cancer. Integrative Cancer Therapies, 17(3), 912–920. https://doi.org/10.1177/1534735418775809
Belin, S., Kaya, F., Duisit, G., Giacometti, S., Ciccolini, J., & Fontés, M. (2009). Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PloS One, 4(2), e4409. https://doi.org/10.1371/journal.pone.0004409
Canadian Cancer Society. (2020). Cancer statistics at a glance. https://www.cancer.ca/en/cancer-information/cancer-101/cancer-statistics-at-a-glance/?region=on
Canadian Cancer Society. (2020). Your emotions and cancer. https://www.cancer.ca/en/cancer-information/living-with-cancer/your-emotions-and-cancer/?region=on
Carosio, R., Zuccari, G., Orienti, I., Mangraviti, S., & Montaldo, P. G. (2007). Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Molecular Cancer, 6, 55. https://doi.org/10.1186/1476-4598-6-55
Cui, B., Luo, Y., Tian, P., Peng, F., Lu, J., Yang, Y., Su, Q., Liu, B., Yu, J., Luo, X., Yin, L., Cheng, W., An, F., He, B., Liang, D., Wu, S., Chu, P., Song, L., Liu, X., Luo, H., … Liu, Q. (2019). Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. The Journal of Clinical Investigation,
129(3), 1030–1046. https://doi.org/10.1172/JCI121685
Doskey, C. M., Buranasudja, V., Wagner, B. A., Wilkes, J. G., Du, J., Cullen, J. J., & Buettner, G. R. (2016). Tumor cells have decreased ability to metabolize H2O2: Implications for pharmacological ascorbate in cancer therapy. Redox Biology, 10, 274–284. https://doi.org/10.1016/j.redox.2016.10.010
Fritz, H., Flower, G., Weeks, L., Cooley, K., Callachan, M., McGowan, J., Skidmore, B., Kirchner, L., & Seely, D. (2014). Intravenous Vitamin C and Cancer: A systematic review. Integrative Cancer Therapies, 13(4), 280–300. https://doi.org/10.1177/1534735414534463
Gröber, U., Holzhauer, P., Kisters, K., Holick, M. F., & Adamietz, I. A. (2016). Micronutrients in oncological intervention. Nutrients, 8(3), 163. https://doi.org/10.3390/nu8030163
Gropper, J.K., Smith, J.L., & Carr, T.P. (2018). Advanced nutrition and human metabolism (7th ed., pp. 303-307, 362). Boston, MA: Cengage Learning
Gupta, P., Tiwari, S., & Haria, J. (2014). Relationship between depression and vitamin C status: A study on rural patients from western Uttar Pradesh in India. Int J Sci Study, 1(4):37-39.
Kessing, L. V., Willer, I. S., & Knorr, U. (2011). Volume of the adrenal and pituitary glands in depression. Psychoneuroendocrinology, 36(1), 19–27. https://doi.org/10.1016/j.psyneuen.2010.05.007
Khanzode, S. D., Dakhale, G. N., Khanzode, S. S., Saoji, A., & Palasodkar, R. (2003). Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Report, 8(6), 365–370. https://doi.org/10.1179/135100003225003393
Klimant, E., Wright, H., Rubin, D., Seely, D., & Markman, M. (2018). Intravenous vitamin C in the supportive care of cancer patients: a review and rational approach. Current Oncology (Toronto, Ont.), 25(2), 139–148. https://doi.org/10.3747/co.25.3790
Levine, M., Padayatty, S. J., & Espey, M. G. (2011). Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Advances in Nutrition (Bethesda, Md.), 2(2), 78–88. https://doi.org/10.3945/an.110.000109
Misono, S., Weiss, N. S., Fann, J. R., Redman, M., & Yueh, B. (2008). Incidence of suicide in persons with cancer. Journal of Clinical Oncology, 26(29), 4731–4738. https://doi.org/10.1200/JCO.2007.13.8941
Moreno-Smith, M., Lutgendorf, S. K., & Sood, A. K. (2010). Impact of stress on cancer metastasis. Future Oncology (London, England), 6(12), 1863–1881. https://doi.org/10.2217/fon.10.142
Padayatty, S. J., Sun, H., Wang, Y., Riordan, H. D., Hewitt, S. M., Katz, A., Wesley, R. A., & Levine, M. (2004). Vitamin C pharmacokinetics: implications for oral and intravenous use. Annals of Internal Medicine, 140(7), 533–537. https://doi.org/10.7326/0003-4819-140-7-200404060-00010
Patak, P., Willenberg, H. S., & Bornstein, S. R. (2004). Vitamin C is an important cofactor for both adrenal cortex and adrenal medulla. Endocrine Research, 30(4), 871–875. https://doi.org/10.1081/erc-200044126
Schlueter, A.K., & Johnston, C.S. (2011). Vitamin c: Overview and update. Journal of Evidence-Based Complementary & Alternative Medicine, 16(1):49-57.
Smith H. R. (2015). Depression in cancer patients: Pathogenesis, implications and treatment (Review). Oncology Letters, 9(4), 1509–1514. https://doi.org/10.3892/ol.2015.2944
Song, H., Saito, E., Sawada, N., Abe, S. K., Hidaka, A., Shimazu, T., Yamaji, T., Goto, A., Iwasaki, M., Sasazuki, S., Ye, W., Inoue, M., & Tsugane, S. (2017). Perceived stress level and risk of cancer incidence in a Japanese population: the Japan Public Health Center (JPHC)-based prospective study. Scientific Reports, 7(1), 12964. https://doi.org/10.1038/s41598-017-13362-8
Stephenson, C. M., Levin, R. D., Spector, T., & Lis, C. G. (2013). Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemotherapy and Pharmacology, 72(1), 139–146. https://doi.org/10.1007/s00280-013-2179-9
Takahashi, H., Mizuno, H., Yanagisawa, A. (2012). High-dose intravenous vitamin c improves quality of life in cancer patients. Pers Med Universe, 1(1), 49-53.
Vickers, A. J., & Cassileth, B. R. (2001). Unconventional therapies for cancer and cancer-related symptoms. The Lancet. Oncology, 2(4), 226–232. https://doi.org/10.1016/S1470-2045(00)00293-X
Vissers, M., & Das, A. B. (2018). Potential mechanisms of action for vitamin c in cancer: Reviewing the evidence. Frontiers in Physiology, 9, 809. https://doi.org/10.3389/fphys.2018.00809
Vollbracht, C., Schneider, B., Leendert, V., Weiss, G., Auerbach, L., & Beuth, J. (2011). Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective,
multicentre, epidemiological cohort study in Germany. In Vivo (Athens, Greece), 25(6), 983–990.
Vollbracht, C., Gündling, P. W., Kraft, K., & Friesecke, I. (2019). Blood concentrations of vitamins B1, B6, B12, C and D and folate in palliative care patients: Results of a cross-sectional study. The Journal of International Medical Research, 47(12), 6192–6205. https://doi.org/10.1177/0300060519875370
Yeom, C. H., Jung, G. C., & Song, K. J. (2007). Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. Journal of Korean Medical Science, 22(1), 7–11. https://doi.org/10.3346/jkms.2007.22.1.7
Zhang, M., Robitaille, L., Eintracht, S., & Hoffer, L. J. (2011). Vitamin C provision improves mood in acutely hospitalized patients. Nutrition (Burbank, Los Angeles County, Calif.), 27(5), 530–533. https://doi.org/10.1016/j.nut.2010.05.016