Abbreviations: Adenosine triphosphate (ATP), Metabolic Correction (MC), Physiological Modulation (PM), Systemic Medicine (SM), Systemic Theory (ST)
.
Introduction
To understand the concepts presented herein, certain terminology must be described. Similar to orthomolecular medicine, physiological modulation (PM) is defined in this context as changes in physiology that improve metabolism, utilizing specific molecules and/or active principles. The intent of PM is to provide a wide variety of metabolically beneficial molecules that have the structure and/or chemical properties needed for a specific physiological situation, increasing available energy and decreasing total entropy of an open biological system. Achieving homeostasis or the balance required for the healthy state is defined herein as a physiological status of balance in which the cells are functioning with all their physiologic capabilities.
PM is based on the Systemic Theory of Living Systems previously described by Olalde, which postulates that the healthy state is based on three synergistic factors: 1) functional organic energy reserve, i.e., the fuel that causes action or movement; 2) active biological intelligence as the regulator that controls and integrates the parts of a living system into a functional unit; and 3) integrity of structure or organization of a group of elements into a functional unit. The second law of thermodynamics states there is a natural tendency of an isolated system to degenerate into a more disordered state. The general change of entropy in a living system consists of internal entropy variations and entropy exchange with the environment. Consistent with this law, Systemic Theory (ST) establishes a common denominator to all sickness, attributing the cause of disease to be an increase in entropy or disorder within the biological system (Olalde, 2005a; Olalde, 2005c).
From this theory, a treatment strategy called Systemic Medicine (SM) is derived. SM largely utilizes PM, which involves identifying and prescribing phytomedicines and/or other natural medications that favor the healthy state by supporting physiological mechanisms. Phytomedicines include adaptogens, which the authors believe may be overlooked and should be incorporated in orthomolecular medicine. Adaptogens are substances of plant origin with polyvalent pharmacological activity that increase an organism’s nonspecific resistance to stress and promote homeostasis at the cellular level (Panossian 2017).
The aim of this article is to provide a scientific explanation of how adaptogens can be useful in orthomolecular medicine for physiological modulation, increasing energy, decreasing entropy and therefore disease, and achieving a healthy state.
.
Discussion
Human Physiological Function and Plant Chemistry
Within a biological system, functional fluctuations occur in response to constantly changing cellular environmental conditions. In order to maintain the proper balance (homeostasis), a series of energy-requiring, orderly reactions must occur. Failure in cellular energy metabolism is a common denominator in chronic degenerative diseases. This failure impacts Huntington’s disease (Browne & Beal, 2004); Alzheimer’s disease (Gabuzda, et al., 1994; Valla, et al., 2001) and aging in general (Toussaint, et al., 1995). Further, intracellular communication, which is a manifestation of biological intelligence, is necessary to maintain cellular organization. The tendency to reach order depends on available energy and information within the system. Stable organization cannot be attained in a living system with insufficient information or energy. Disease, therefore, may be defined as a state of disorganization, i.e. higher organic entropy, corresponding with a low energy/informational status of the system (Olalde, 2005a).
A true healthy state facilitates and promotes energy production, formation of structures, tissue growth and repair, cell differentiation, detoxification and production of the necessary chemicals (hormones and neurotransmitters, cytokines, growth factors) in a delicate dynamic balance (Gonzalez, et al. 2018). The pathological state (physiological entropy) usually results from multiple causes and therefore, a synergistic approach is required to correct this state. The Metabolic Correction (MC) concept can supply the necessary cofactors in the right form and dose to influence cellular biochemistry (Gonzalez & Miranda-Messari, 2012; Gonzalez, et al. 2015); PM can function as a synergistic partner to MC by providing key exogenous molecules that produce, replicate or enhance a necessary physiological effect to help restore normal physiology favoring the healthy state.
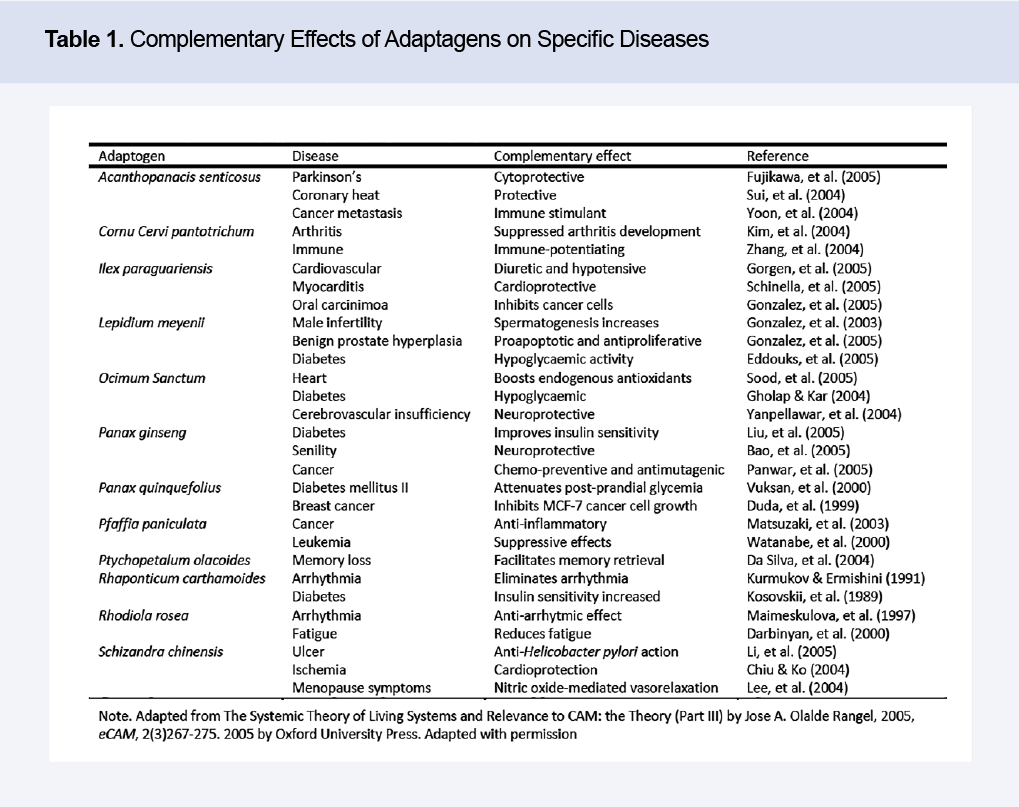
The application of PM involves classifying and applying botanicals according to a specific type: energy stimulators; biological intelligence modulators and organizational (structural and functional) enhancers. Adaptogenic botanicals contain compounds that possess the structure and/or chemical properties needed for these actions. The therapeutic benefits of adaptogens range from anti-viral to anti-inflammatory, and increased adenosine triphosphate (ATP) production (See Table 1). Note that each of the adaptogens listed in Table 1 also have ATP synthesis and re-synthesis as a main effect making them potential therapies for energy-deficiency related diseases, such as Parkinson’s, Huntington’s, Alzheimer’s, multiple sclerosis, and in general, all chronic degenerative diseases. Adaptogens such as Rhodiola rosea and Eleutherococcus senticosus can also have beneficial effects in fundamental biological processes such as oxidative phosphorylation and nitric oxide production. The Golden Rule of Therapeutics described by Olalde (2005b) recommends at least one of the energy adaptogens listed in Table 1 be used in chronic degenerative disease protocols.
Adaptogenic plants have been used for centuries in Chinese, African, Tibetan, Ayurvedic, and Cherokee medicine to restore balance and health (Antoshechkin, 2001). The term “adaptogen” was originally coined in 1947 when it was recognized that “adaptogens” are able to increase a non-specific resistance of the organism to stress factors and thereby promote its adaptation to stressful external conditions without causing harm. The concept of adaptogen has been modified over the years. In the 1990s, Wagner, Wikman, and Panossian performed many studies on adaptogens and called them “natural bioregulators.”. In 1998, the FDA defined an adaptogens as a new kind of metabolic regulator that has been proved to help in environmental adaptation and to prevent external harms (Lian-ying, et al. 2018). Studies have shown that adaptogens enhance energy mechanisms such as the biosynthesis of ATP (Panossian, et al. 1999 a, b, c).
Adaptogens as a Physiological Modulation Mechanism
Adaptogens normalize endocrine function through numerous broad and non-specific actions, and increase overall resistance to stressors; they exert unique actions on the adrenal system that tend to promote resistance to the negative effects of stress on the body; they support the activation and synchronization of both the neuroendocrine system and cellular energy metabolism, that are reduced by illness, physical/mental fatigue and aging (Gonzalez, et al., 2018). Adaptogens exhibit polyvalent beneficial effects against chronic inflammation, atherosclerosis, neurodegenerative cognitive impairment, metabolic disorders, cancer, and other aging-related diseases (Panossian, 2017).
Several theories have been proposed to explain the effects of adaptogens. One theory proposed by Davydov and Krikorian (2000), stated that adaptogens function primarily due to their antioxidant and free radical scavenging capacity. This theory is only partially accurate since this theory falls short of explaining the full effects of these medicinals. Adaptogens enhance normal physiological functions rather than pushing the body toward a specific outcome. Thus, the administration of adaptogens can result in the stabilization of physiological processes and promotion of homeostasis, leading to the healthy state.
.
.
Bidirectional Action of Adaptogens
To normalize body function, adaptogens are capable of producing a bidirectional effect on physiological function. Adaptogens can either downregulate the activity of hyperfunctioning systems or strengthen activity of low-functioning systems. Panax ginseng’s ginsenosides Rg1 and Rb1 are good examples of this bidirectionality. Rg1 can stimulate angiogenesis, mitosis, and the nervous system while Rb1 has the opposite effect. Ginseng also has hypertensive and hyptotensive properties. The directional decision is left to biological intelligence based on the system’s needs (Han, et al., 2005; Ogawa-Ochia & Kawasaki, 2019; Olalde, 2005c; Scott, et al., 2001).
Adaptogenic herbs stimulate and balance several systems, most notably the neuroendocrine and immune systems. Adaptogens can have numerous pharmacological effects and indications that can help the body reach the state of homeostasis. Chemically, the adaptogens are typically either complex phenolics or tetracyclic triterpenoids (Panossian & Wikman G, 2010). The phenolics are presumed to act via the hypothalamic-pituitary-adrenal (HPA) axis, while the terpenoids are presumed to interact with the sympathoadrenal system (SAS). The HPA axis and SAS are anatomically and functionally interconnected. Some plants such as Eleutherococcus, have the potential to modulate both the HPA axis and SAS due to multiple bioactive compounds (Panossian, 2017).
Expanded Explanation of Adaptogens: A Mechanistic View
Adaptogens work in a multi‐targeted, multi‐channel, network‐like manner, sharing a number of different receptors, and acting mainly by affecting the hypothalamic–pituitary–adrenal axis. They can also affect the immune, nervous, and endocrine systems, and therefore, the entire body. Adaptogens also affect gene expression, acting to deregulate genes that code for proteins that play important roles in behavioral, cognitive, and age-associated disorders (Lian-ying, et al. 2018; Panossian, 2017).
A number of studies have suggested several mechanisms of actions related the beneficial stress-protective effect of adaptogens and regulation of aging and disease pathology of age-related disease. Key players in mediating the effects include corticol, neuropeptide Y (NPY), heat shock factor-1(HSF1), heat shock proteins (HSP25, HSP27, HSP70, and HSP 72), stress-activated c-Jun Nterminal protein kinase (JNK1), Forkhead Box O (FOXO) transcription factor DAF-16, cortisol and nitric oxide (NO), with no single contribution that can be estimated with any degree of certainty. The molecular targets HSF1/HSP70 and FOXO are related to complimentary longevity pathways. Whereas chemicals used to induce HSP70 are typically cytotoxic and unsuitable for patients more susceptible to stress, such as the elderly, PM of these pathways using adaptogens to delay the onset of neurodegenerative and age-related diseases is a viable therapeutic solution. (Panossian & Wikman, 2009; Panossian, 2017).
The pharmacology of adaptogens is that of network pharmacology. Multiple molecular networks are involved that coordinate both intracellular and extracellular signaling. The metabolic regulation of homeostasis by adaptogens at the cellular and systems levels is associated with multiple targets. Network pharmacology is a distinctive new approach that involves application of network analysis to determine the set of molecules capable of targeting specific characteristics of a disease. By addressing the true complexity of disease and by seeking to harness the ability of compounds to influence many different pathways, network pharmacology differs from conventional drug discovery approaches which have generally been based on highly specific targeting of a single pathway or protein. Network pharmacology has the potential to provide treatments for complex diseases, chronic conditions, and syndromes where conventional approaches have often been disappointing (Panossian, 2017).
The plethora of terpenoid and phenolic compounds in adaptogens most likely explains their multi-target effects and polyvalent pharmacological actions. This unique set of synergistic, complementary compounds are capable of oxidation–reduction actions depending on the system’s needs, guided by its energy status. Other compounds in adaptogens such as alkaloids and flavonoids have anti-viral, anti-inflammatory, and antioxidant properties. Any adaptogenic plant has dozens of active principles and adaptogens display their greatest efficacy in the form of extracts containing a combination of several active substances from a single plant species. Additionally, combinations of different adaptogens can also act synergistically without adverse effects, provided that they are administered by trained professionals who maintain appropriate patient supervision (Abdimov, et al. 2003; Bucci, 2000; Olalde, 2005c; Panossian, 2003).
The principles of systems biology and pharmacological networks appear to be more suitable for conceptualizing adaptogen function. Understanding the mechanisms of action of adaptogens requires a holistic approach. A one-drug, one-indication paradigm is not suitable for adaptogens. Nor can the mechanistic aspects of the physiological notion of “adaptability” and the adaptogenic activity of adaptogens be adequately described using the reductionist model or single receptor-based pharmacology (Panossian, 2017).
.
Conclusion
If a reduction in illness is to be achieved, energy must be increased, and entropy reduced. A comprehensive way of accomplishing this is administering negative entropy, or order, through PM using adaptogens that stimulate the production of energy and provide survival information to the immune, neuroendocrine and cellular systems. A more complete result or system optimization can be achieved by complementing the actions of PM with MC via the provision of necessary cofactors and coenzymes that guide cellular metabolism. These two therapeutic physiologic balancing actions may activate biological intelligence and create the necessary environment to favor the healthy state. Protocols combining MC (diet and scientific supplementation or orthomolecular therapy) and PM (botanicals/adaptogens and systemic therapy) may reduce medication requirements and adverse side effects, and improve treatment outcomes for chronic, degenerative and age-related diseases.
.
References
Abidov M, Crendal F & Grachev S. (2003) Effect of extracts from Rhodiola rosea and Rhodiola crenulata (Crassulaceae) roots on ATP content in mitochondria of skeletal muscles. Bull Exp Biol Med, 136: 585–7.
Antoshechkin, A. (2001) The Primary Adaptogens. Clearwater: Ceptima Publishing Co. Inc.
Bao HY, Zhang J, Yeo SJ, Myung CS, Kim HM, Kim JM, . . . Kang JS. (2005) Memory enhancing and neuroprotective effects of selected ginsenosides. Arch Pharm Res 28:335–42
Browne SE & Beal MF. (2004) The energetics of Huntington’s disease. Neurochem Res 29(3):531-46.
Bucci LR. (2000) Selected herbals and human exercise performance. Am J Clin Nutr, 7: 624S–36S.
Chiu PY & Ko, K.M. (2004) Schisandrin B protects myocardial ischemia- reperfusion injury partly by inducing Hsp25 and Hsp70 expression in rats. Mol Cell Biochem 266:139–44.
Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G & Wagner H. (2000) Rhodiola rosea in stress induced fatigue—a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine 7:365–71.
da Silva AL, Piato AL, Bardini S, Netto CA, Nunes DS & Elisabetsky E. (2004) Memory retrieval improvement by Ptychopetalum olacoides in young and aging mice. J Ethnopharmacol 95:199–203.
Davydov M & Krikorian AD. (2000) Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an Adaptogen: A Closer Look, J Ethnopharmacol 72:345-393.
Duda RB, Zhong Y, Navas V, Li MZ, Toy BR & Alavarez JG. (1999) American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth. J Surg Oncol 72:230–9.
Eddouks M, Magharani M, Zeggwagh NA & Michel JB. (2005) Study of the Hypo-glycaemic activity of Lepidium sativum L. aqueous extract in normal and diabetic rats. J Ethnopharmacol 97:391–5.
Fujikawa T, Miguchi S, Kanada N, Nakai N, Ogata M, Suzuki I & Nakashima K. (2005) Acanthopanax senticosus Harms as a prophylactic for MPTP-induced Parkinson’s disease in rats. J Ethnopharmacol 97:375–81.
Gabuzda D, Busciglio J, Chen LB, Matsudaira P & Yankner BA. (1994) Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentiall amyloidogenic derivative. J Biol Chem 269, 13623-13628.
Gholap S, Kar A. (2004) Hypoglycaemic effects of some plant extracts are possibly mediated through inhibition in corticosteroid concentration. Pharmazie 59:876–8.
Gonzales GF, Rubio J, Chung A, Gasco M & Villegas L. (2003) Effect of alcoholic extract of Lepidium meyenii (Maca) on testicular function in male rats. Asian J Androl 5:349–52.
Gonzales GF, Miranda S, Nieto J, Fernandez G, Yucra S, Rubio J, . . . Gasco M. (2005) Red Maca (Lepidium meyenii) reduced prostate size in rats. Reprod Biol Endocrinol 3:5.
Gonzalez MJ & Miranda-Massari JR (2012) Metabolic Correction: A Functional Explanation of Orthomolecular Medicine. J Orthomolec Med 2: 13-20.
Gonzalez MJ, Miranda-Massari JR, Hickey S, Duconge J, Allende-Vigo MZ, Jimenez Ramirez FJ, . . . Berdiel MJ (2015) Metabolic Correction: A Functional Biochemical Mechanism against Disease. Part I: Concept and Historical Background. P R Health Sci J 34: 3-8
Gonzalez MJ, Olalde J, Rodriguez JR, Rodriguez D & Duconge J. (2018) Metabolic Correction and Physiologic Modulation as the Unifying Theory of the Healthy State: The Orthomolecular, Systemic and Functional Approach to Physiologic Optimization. J Orthomolec Med 33(1).
Gonzalez de Mejia E, Song YS, Ramirez-Mares MV, & Kobayashi H. (2005) Effect of yerba mate (Ilex paraguariensis) tea on topoisomerase inhibition and oral carcinoma cell proliferation. J Agric Food Chem 53: 1966–73.
Gorgen M, Turatti K, Medeiros AR, Buffon A, Bonan CD, Sarkis JJ & Pereira JS. (2005) Aqueous extract of Ilex paraguariensis decreases nucleotide hydrolysis in rat blood serum. J Ethnopharmacol 97:73–7.
Han, K, Shin IC, Choi KJ, Yun YP, Hong JT & Oh KW. (2005) Korea red ginseng water extract increases nitric oxide concentrations in exhaled breath. Nitric Oxide 12(3):159-62.
Kim KS, Choi YH, Kim KH, Lee YC, Kim CH, Moon SH. . . . Park YG. (2004) Protective and anti-arthritic effects of deer antler aqua-acupunture (DAA), inhibiting dihydroorotate dehydrogenase, on phosphate ions-mediated chondrocyte apoptosis and rat collagen-induced arthritis. Int Immunopharmacol 4:963–73.
Kosovskii MI, Syrov VN, Mirakhmedov MM, Katkova SP & Khushbaktova ZA (1989) The effect of nerobol and ecdysterone on insulin-dependent processes linked normally and in insulin resistance. Probl Endokrinol (Mosk) 35:77–81.
Kurmukov AG & Ermishina OA. (1991) The effect of ecdysterone on experimental arrhythmias and changes in the hemodynamics and myocardial contractility induced by coronary artery occlusion. Farmakol Toksikol 54:27–9.
Lee YJ, Cho JY, Kim JH, Park WK, Kim DK & Rhyu MR. (2004) Extracts from Schizandra chinensis fruit activate estrogen receptors: a possible clue to its effects on nitric oxide-mediated vasorelaxation. Biol Pharm Bull 27:1066–9.
Li Y, Xu C, Zhang Q, Liu JY & Tan RX. (2005) In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol 98:329–33.
Lian-Ying L, Yi-Fan H, Li L, Hong M, Yin-Mao D, Fan Y & Peigen X. (2018) A preliminary review of studies on adaptogens: comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chin Med 13:57.
Liu TP, Liu IM & Cheng JT. (2005) Improvement of insulin resistance by Panax ginseng in fructose-rich chow-fed rats. Horm Metab Res 37:146–51.
Maimeskulova LA, Maslov LN, Lishmanov IB & Krasnov EA. (1997) The participation of mu-, delta- and kappa-opioid receptors in the realization of the anti-arrhythmia effect of Rhodiola rosea. Eksp Klin Farmakol 60:38–9.
Matsuzaki P, Akisue G, Salgado Oloris SC, Gorniak SL & Zaidan Dagli ML. (2003) Effect of Pfaffia paniculata (Brazilian ginseng) on the Ehrlich tumor in its ascitic form. Life Sci 74:573–9.
Ogawa-Ochiai K & Kawasaki K. (2019) Panax ginseng for Frailty-Related Disorders: A Review. Frontiers in nutrition, 5, 140.
Olalde Rangel JA. (2005a) The systemic theory of living systems and relevance to CAM: part I: the theory. Evid Based Complement Alternat Med 1: 13–8.
Olalde Rangel JA. (2005b) The systemic theory of living systems and relevance to CAM: the theory (part II). Evid Based Complement Alternat Med 2: 129–37.
Olalde Rangel JA. (2005c) The systemic theory of living systems and relevance to CAM. Part III: the theory. Evid Based Complement Alternat Med 2: 267–75.
Panossian A. (2003) Adaptogens, Tonic Herbs for Fatigue and Stress. Altern & Complement Ther 9(6):327-331.
Panossian A. (2017) Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann N Y Acad Sci 1401(1):49-64.
Panossian A, Wikman G & Wagner H. (1999a) Plant adaptogens III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine 6:287–300.
Panossian A, Gabrielian E & Wagner H. (1999b) On the mechanism of action of plant adaptogens with particular references on cucurbitacin R diglucoside. Phytomedicine 6:147–55.
Panossian A, Oganessian A, Ambartsumian M, et al. (1999c) Effects of heavy physical exercise and adaptogens on nitric oxide content in human saliva. Phytomedicine 6:17–26.
Panossian A & Wikman G. (2009) Evidence-Based Efficacy of Adaptogens in Fatigue, and Molecular Mechanisms Related to their Stress-Protective Activity. Current Clinical Pharmacology 4(3),198.
Panossian A & Wikman G. (2010) Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress—Protective Activity. Pharmaceuticals 3: 188-224.
Panwar M, Kumar M, Samarth R, Kumar A. (2005) Evaluation of chemo-preventive action and antimutagenic effect of the standardized Panax ginseng extract, EFLA 400, in Swiss albino mice. Phytother Res 19:65–71.
Schinella G, Fantinelli JC & Mosca SM. (2005) Cardioprotective effect of Ilex paraguariensis extract: evidence for a nitric oxide-dependent mechanism. Clin Nutr 24:360–6.
Scott GI, Colligan PB, Ren BH & Ren J. (2001) Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. British Journal of Pharmacology, 134(6), 1159-65.
Sood S, Narang D, Dinda AK & Maulik SK. (2005) Chronic oral administration of Ocimum sanctum Linn. augments cardiac endogenous antioxidants and prevents isoproterenol-induced myocardial necrosis in rats. J Pharm Phramacol 57:127–33.
Sui DY, Qu SC, Yu XF, Chen YP & Ma XY. (2004) Protective effect of ASS on myocardial ischemia-reperfusion in rats. Zhongguo Zhong Yao Za Zhi 29:71–4.
Toussaint O, Michiels C, Raes M & Remacle J. (1995) Cellular aging and the importance of energetic factors. Exp Gerontol 30:1–22.
Valla J, Berndt JD & Gonzalez-Lima F. (2001) Energy Hypometabolism in Posterior Cingulate Cortex of Alzheimer’s Patients: Superficial Laminar Cytochrome Oxidase Associated with Disease Duration. Journal of Neuroscience 21 (13) 4923-4930.
Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U & Xu Z. (2000) American ginseng (Panax quinquefolius L) reduces postprandial glycemia in non-diabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med 160:1009–13.
Watanabe T, Watanabe M, Watanabe Y & Hotta C. (2000) Effects of oral administration of Pfaffia paniculata (Brazilian ginseng) on incidence of spontaneous leukemia in AKR/J mice. Cancer Detect Prev 24:173–8.
Yanpallewar SU, Rai S, Kumar M, Acharya SB. (2004) Evolution of antioxidant and neuroprotective effect of Ocimum sanctum on transient cerebral ischemia and long-term cerebral hypoperfusion. Pharmacol Biochem Behav 79:155–64.
Yoon TJ, Yoo YC, Lee SW, Shin KS, Choi WS, Hwang SH. . . . Park WM. (2004) Anti-metastasic activity of Acanthopanacis senticosus extract and its possible immunological mechanism of action. J Ethnopharmacol 93:247–53.
Zhang L, Wang Y, Wang LZ & Gao XM. (2004) Immunopotentiating effect of a ‘Yang’-promoting formula of traditional Chinese medicine on aged female BALB/c mice. Phytother Res 18:857–61.


