Introduction
The importance of the consumption of the vitamin C for human health is well known, as humans do not have gulonolactone oxidase, the enzyme required for vitamin C biosynthesis. Many studies demonstrate that adequate availability of vitamin C is necessary for stimulation of certain enzymes, collagen biosynthesis, hormonal activation, detoxification of histamine, proper immune functioning, suppression of virus replication, suppression of inflammation, and in the process of healing many chronic diseases (Levy, 2012).
Vitamin C deficiency is common in patients with many chronic conditions, including cancer (Schorah, Downing, Piripitsi, Gallivan, et al., 1996).
The oral route is the most convenient way to administer nutraceuticals, including vitamin C. However, it has been shown that plasma ascorbic acid levels following oral administration are limited and have maximum concentration around 220 uM, depending on the dose administered (Levine, Conry-Cantilena, Wang, et al. 1996; Padayatty, Sun, Wang, Riordan, et al., 2004). Ascorbic acid bioavailability is largely determined by rates of intestinal absorption and further influenced by renal reabsorption and excretion (Levine, Padayatty, & Espey, 2011; Levine, Rumsey, Daruwala, Park, & Wang, 1999; Wang, Mackenzie, Tsukaguchi, et al., 2000). As the result, it is difficult to increase plasma and tissue concentrations by oral intake of ascorbic acid. For treatment of certain conditions such as cancer, arthritis, viral infections and others, intravenous administration of ascorbate can produce concentrations that cannot be achieved orally (Riordan, Riordan, & Casciari, 2000).
Now a different method of administration of ascorbic acid is proposed by utilizing liposomal encapsulation (Panagiotou, & Fisher, 2013). The goal of the utilizing encapsulated ascorbic acid is to improve the penetration of various biological membranes (mucosa, epithelium, or endothelium), with further diffusion through the plasma membrane and finally gaining access to the appropriate cells.
Liposomes were first discovered by Bangham in 1965, and the first liposomal pharmaceutical product, Doxil, received FDA approval in 1997 for the treatment of chemotherapy refractory AIDS-related Kaposi’s sarcoma (Deamer, 1978; Szoka, & Papahadjopoulos, 1978). During the past years liposomes have received a lot of attention as pharmaceutical carriers of great potential. Liposomes are able to encapsulate lipophilic or hydrophilic substances within their lipidic layers or in their inner aqueous compartment. Advantages of liposomal encapsulation include accelerated intestinal absorption, increased stability of the pharmaceuticals, protection of the gut from potentially irritating agents, and greater bioavailability of the pharmaceuticals (Kraft, Freeling, Wang, & Ho, 2014). Studies have shown that liposomal formulation (method of preparation, size, and type of encapsulated drug) has a great influence on the efficiency of drug delivery (Otake, Imura, Sakai, & Abe, 2001; Uhumwangho & Okor, 2005; Jiskoot, Teerlink, Beuvery, & Crommelin, 1986).
Currently there are about 12 liposome-based drugs approved for clinical use and more are in various stages of clinical trials (Chang & Yeh, 2012; Torchilin, 2005). Most liposomal drug formulations are approved for intravenous application. Other administration routes such as intramuscular delivery have also been approved. Oral delivery of drugs has been examined; however, this route of administration is more troublesome due to the potential for liposomal breakdown following exposure to bile salts (Shaji & Patole, 2008). The studies of the oral liposomal formulations of ascorbic acid include a study published by Devis and coworkers (Davis, Paris, Beals, et al., 2016), in which the authors compared oral absorbance of liposomal ascorbic acid to oral absorbance of a non-encapsulated form, and intravenous delivery. On four separate randomly ordered occasions, 11 men and women were administered an oral placebo, or 4 g of vitamin C via oral, oral liposomal, or intravenous delivery. The data indicate that oral delivery of 4 g of vitamin C encapsulated in liposomes produces circulating concentrations of vitamin C that are greater than non-encapsulated oral, but less than achieved by intravenous administration. In addition, liposomal vitamin C provides protection from ischemia reperfusion-mediated oxidative stress that is similar to the protection provided by un-encapsulated oral and intravenous administrations. The levels of ascorbate that were achieved in plasma after 4 grams vitamin C were 1419 uM for intravenous ascorbate, 124 uM for un-encupsulated and 170 uM for encapsulated ascorbate.
In the paper of Hickey (Hickey, Roberts, & Miller, 2008), the highest achievable plasma ascorbic acid concentrations by oral intake of liposomal formulations of ascorbic acid were determined for dosages of 5 grams, 20 grams and 36 grams. Two subjects took part in this study, and blood plasma measurements were obtained over the first 6 hours of absorption. Circulating concentrations of vitamin C following oral delivery of 5 g of ascorbic acid encapsulated in liposomes were compared with concentrations following 5 g of un-encapsulated ascorbic acid and showed that both produced similar response curves. Plasma levels following a 36 g oral dose of liposomal ascorbic acid resulted in peak plasma levels in the region of 400 uM/L with delayed the peak plasma level and broader response.
In our study we compared the circulating concentrations of ascorbic acid and dehydroascorbic acid in plasma and peripheral blood mononuclear cells following oral ingestion of ascorbic acid encapsulated within liposomes (25 grams) to injection of the same concentrations of ascorbic acid intravenously.
Design
We studied the concentrations of reduced and oxidized ascorbate in serum and cells after 25 grams of ascorbate taken orally in liposomal form and after intravenous injections of 25 grams in two adult healthy humans. The experimental protocol conformed to the standards set by the Declaration of Helsinki of 1975, as revised in 1983, and was approved by the IRB at the Riordan Clinic. The nature, purpose, and risks of the study were explained to each research participant before written informed consent was obtained.
As the IVC infusion lasts 40-60 min, one gram of liposomal ascorbate was taken at two minute intervals for 50 minutes. Venous blood was sampled at baseline, at the end of IVC, and 3 hours after total liposomal dosage, as the data in the study (Davis, Paris, Beals, et al., 2016) show that the maximal concentration of vitamin C in plasma is reached 3 hours after oral intake of liposomal ascorbate.
The IVC injections were performed according to Riordan clinic protocol: 250 cc sterile water, 25 g ascorbic acid (500 mg/mL), 2 cc MgCl (200mg/mL), and 10 cc NaBicarb (8.4%). One package of lyposomal ascorbic acid consisted: Vitamin C (as sodium ascorbate) 1,000 mg (1,666%), sodium (as sodium ascorbate) 120 mg (5%), essential Phospholipids (EPL) 1,000 mg (from soy lecithin), and phosphatidylcholine (PC) 500 mg.
Method of Measurements
The level of ascorbate and dehydroascorbic acid was measured by a method based on Cayman’s ascorbic acid assay and the original study of Vislisel and coworkers (Vislisel, Schafer, & Buettner, 2007). This method of analysis of total intracellular ascorbate and oxidized ascorbate is based on the well-known condensation reaction of dehydroascorbic (DHA) with o-phenylenediamine (OPDA) to form a highly colored product that can be detected by fluorescence. In the assay, ascorbate must be oxidized to DHA. This traditionally has been done with ascorbate oxidase. In this assay the oxidation of ascorbate is accomplished by using Tempol. The level of ascorbate in plasma and cells was analyzed by fluorescence (Fluorolog-3, Jobin Yvon Inc) by exciting at wavelength range of 340-350 nm and measuring an emission peak at wavelengths 420 nm – 430 nm.
Sample Preparation
Plasma Whole blood was collected in heparin tubes and centrifuged at 4°C, 1000 g, for 10 min. The upper plasma layer was transferred to a new centrifuge tube and one part of plasma was mixed with four parts of MeOH/H2O/DTPA (90:7.5:2.5, v/v/v). The mixture was incubated on ice for 10 min to precipitate the protein. The samples were centrifuged at 4°C, 12000 g, for 10 min. Supernatants were stored at -80°C until analysis.
PBMCs PBMCs were separated by Ficoll-Pague procedure according to the company’s recommendations. Blood was analyzed as soon as possible and kept on rocker to prevent coagulation. Cells were washed twice with PBS, and the cell count was performed by standard lab procedure by hemocytometer. Cell pellets (3-5 million cells) were frozen at -80°C to rupture the membranes. After freezing during 30 min, cell pellets are thawed and extracted with 200 μL MeOH/H2O/DTPA (75/22.5/ 2.5, v/v/v), incubated on ice for 10 min, and then centrifuged at 12000 g for 5 min. Supernatant can be stored at -80°C until analysis. Standard deviation of ascorbate measurements in plasma and cells was in the range of 10%-15%.
Reagents Ascorbic acid was purchased from Sigma, sodium acetate buffer was from Cayman Chemicals, diethylenetriaminepentaacetic acid (DETAPAC or DTPA), ophenylenediamine dihydrochloride (OPDA), and 4-hydroxy-2,2,6,6- tetramethylpiperidinyloxy (Tempol; CAS No: 2226-96-2) are from Sigma-Aldrich (St. Louis, MO); methanol was from Fisher Scientific (Fair Lawn, NJ).
Results
Plasma Ascorbate and DHA Concentrations
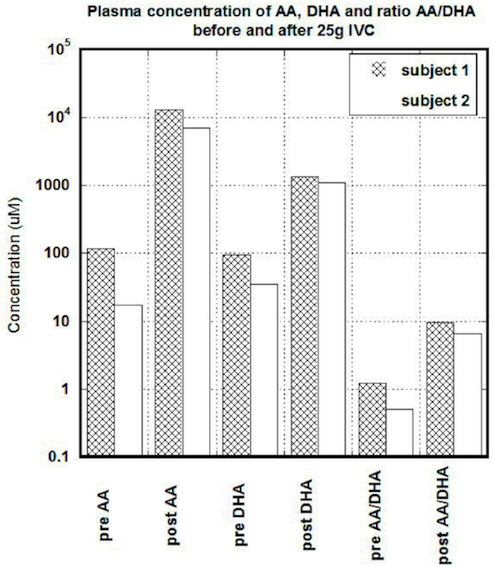
In first subject, the serum concentration of ascorbate after 25 grams IVC was increased from 118 uM to 13,200 uM (112 times). Concentration of DHA was changed from 95 uM to 1215 uM (14 times). The ratio of ascorbate to DHA was increased from baseline 1.24 to 9.6. In the second subject, the baseline concentration of ascorbate in blood was 35 uM and increased to 7071 uM. DHA was raised from 35 uM to 1090 uM, and the ratio of ascorbate to DHA was changed from 0.5 to 6.5.
Results of measurements before and after IVC for both subjects are shown in Figure 1. Data are presented for baseline and after treatment levels of ascorbate, DHA, and the ratio of reduced to oxidized ascorbate.
Figure 1
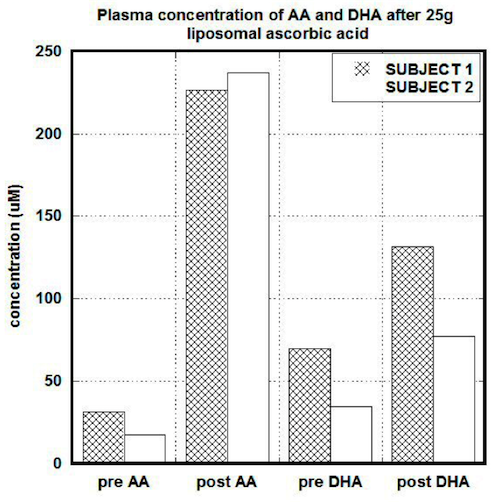
After liposomal intake of 25g of ascorbate (1 gram every 2 minutes) concentrations in serum in the first subject were increased 7 times (from 31.7 uM to 226 uM) for ascorbate and 1.9 times (from 69 uM to 131uM) for DHA. The ratio AA/DHA was changed from 0.5 to 1.7. In the second subject the pre and post values after liposomal ascorbate uptake were 30 uM and 237 uM for ascorbate, 35 uM and 77.2 uM for DHA and 0.5 and 3 for the ratio of ascorbate to DHA. Plasma concentrations after liposomal uptake for both subjects are shown in Figure 2.
Figure 2
Ascorbate and DHA concentrations in PBMCs
As shown by many research studies, PBMCs concentrate ascorbic acid in high levels and against a concentration gradient, suggesting that this substance has an important role in these cells’ physiological function (Leibovitz & Siegel, 1978; Prinz, Bortz, Bregin, & Hersch, 1977).
In our study, we compared the uptake of ascorbic acid by cells after intravenous and liposomal ascorbic acid supplementation. The results of our measurements for two patients are shown in Figures 3(a, b).
Figure 3a
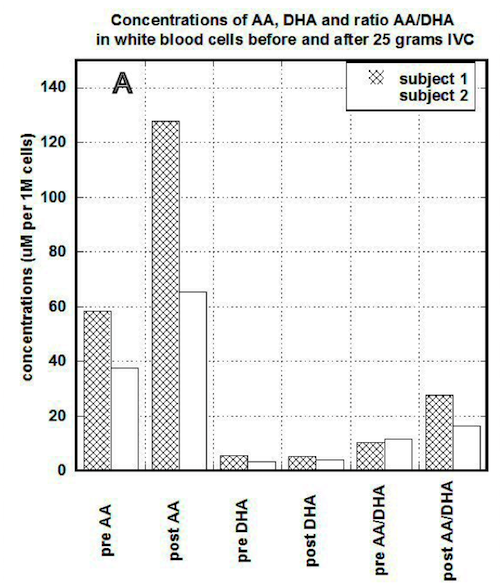
Figure 3b
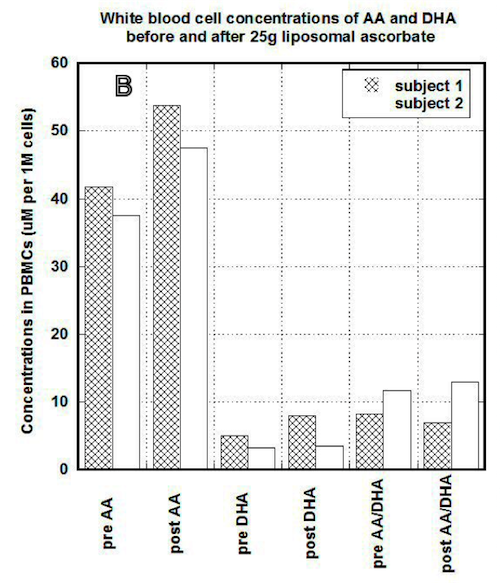
In PBMCs, IVC injections increased concentration of ascorbate from 60 um per 1 million cells (um/M) to 120 um/M for first subject and from 38 um/M to 60 um/M for the second subject. After the same dosage of liposomal ascorbate the intracellular concentration of ascorbate was increased from 37 um/M – 42 um/M to 48 um/M-54 um/M.
Discussion
As it was described before, liposomal formulations were developed for improvement of the delivery systems intended to protect drugs/supplements from pH changes, digestive enzymes, and bile salts in order to improve their absorption.
The purpose of the liposomal coating of ascorbic acid is to increase the gastrointestinal absorption by the mechanism of dissolving liposomes through the lining of the stomach and enhancing penetration into the bloodstream and cell walls. This should result in a higher level of ascorbate absorption into the body than through non-encapsulated oral administration.
According to our data, concentration of ascorbate in plasma, after administration of 25 grams intravenous vitamin C, increased from 112 to 140 times. The oral liposomal intake of 25 grams of ascorbate resulted in increasing concentrations in plasma seven times and reached in an average value 230 uM. According to another study (Hickey, Roberts, & Miller, 2008), this level in plasma can be achieved by less doses of standard ascorbic acid supplementation. The concentration of dehydroascorbic acid in plasma was raised 14 -20 times after intravenous ascorbate and 2 times after liposomal ascorbate.
The injection of 25 grams ascorbate intravenously resulted in the increase of ascorbic acid concentration in cells 1.7-2.2 times. Supplementation by the same dosage of liposomal ascorbate resulted in a slight increase in the intracellular levels of ascorbate (25%-30%).
Our data demonstrated that both routes of ascorbic acid supplementation changed the content of intracellular ascorbic acid, but did not change the level of DHA, indicating that if mononuclear cells take up DHA by a sodium independent transporter systems, in addition of an intake of ascorbate by a sodium dependent transporter, within the cells DHA is immediately reduced back to ascorbate.
The reason for a great difference in the concentrations of ascorbate delivered intravenously vs encapsulated in liposomes is that the oral administration of liposomes requires high stability. According to the studies (Rogers & Anderson, 1998; Taira, Chiaramoni, Pecuch, & Alonso-Romanowski, 2004; Hermida, Sabés-Xamaní, & Barnadas-Rodríguez, 2009), the environment of the gastrointestinal tract can disrupt a large percentage of these carriers. The concerted action of duodenal enzymes and bile salts destroy the lipid bilayer of most liposomes, especially those composed of unsaturated lipids, and consequently the transported drug/supplement, either encapsulated or bound at the membrane, is released before reaching the absorption site (Chiang & Weiner, 1987; Dapergolas & Gregoriadis, 1976; Patel & Ryman, 1976, Rowland & Woodley, 1980; O’Connor, Wallace, Iwamoto, Taguchi, Sunamoto, 1985).
Hydrolysis, oxidation of phospholipids and liposome aggregation are also common causes of liposome instabilities (Allen & Moase, 1996; Immordino, Dosio, & Cattel, 2006; Shaji & Patole, 2008). The hydrolytic degradation of phosphatidylcholine can be increased by addition of an antioxidant, in our case ascorbic acid. Also efficacy of absorption depends on the size of particles, as large liposomes cannot be taken up by the M-cells (microfold cells), and small liposomes cannot elicit efficient internalization and absorption.
In summary, our data support the fact that pharmacological concentrations of ascorbate in blood can be achieved only by intravenous administration of ascorbate.
Competing Interests
The author declares that she has no competing interests.
References
Allen, TM, Moase, EH (1996). Therapeutic opportunities for targeted liposomal drug delivery. Adv Drug Deliv Rev, 21, 117-133.
Chang, HI, Yeh, MK. (2012). Clinically-Proven Liposome-Based Drug Delivery: Formulation, Characterization and Therapeutic Efficacy. Int J Nanomedicine, 7, 49-60.
Chiang, C.M., Weiner, N. (1987). Gastrointestinal uptake of liposomes. II. In vivo studies. Int J Pharm, 40, 143–149.
Dapergolas, G., Gregoriadis, G. (1976). Hypoglycaemic effect of liposome- entrapped insulin administered intragastrically into rats. Lancet, 2, 824–827.
Davis, J.L., Paris, H.L., Beals, J.W., Binns, S.E., Giordano, G.R., Scalz,o R.L., Schweder, M.M., Blair, E., Bel, C. (2016). Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia–Reperfusion. Nutrition and Metabolic Insights, 9, 25–30.
Deamer, Q.W. (1978). Preparation and properties of ether-injection liposomes. Ann N Y Acad Sci, 308, 250–258.
Hermida, L.G., Sabés-Xamaní, M., Barnadas-Rodríguez, R. (2009). Combined strategies for liposome characterization during in vitro digestion. Journal of Liposome Research, 19(3), 207–219.
Hickey, S., Hilary, J., Roberts, H.J., Miller, N.J.(2008). Pharmacokinetics of oral vitamin C. Journal of Nutritional & Environmental Medicine. 11:08:27.
Immordino, M.L., Dosio, F., Cattel, L. (2006). Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine, 1, 297-315.
Jiskoot, W., Teerlink, T., Beuvery, E.C., Crommelin, D.J. (1986). Preparation of liposomes via detergent removal from mixed micelles by dilution. The effect of bilayer composition and process parameters on liposome characteristics. Pharm Weekbl Sci, 8(5), 259–265.
Kraft, J.C., Freeling, J.P., Wang, Z., Ho, R.J. (2014). Emerging research and clinical develop¬ment trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci,103(1), 29–52.
Leibovitz, B., Siegel, B.V. (1978). Ascorbic acid, neutrophil function, and the immune response. Int J Vitam Nutr Res, 48(2), 159-164.
Levine, M., Conry-Cantilena, C., Wang, Y, et al. (1996). Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. PNAS, 93, 3704-3709.
Levine, M., Padayatty, S.J., Espey, M.G. (2011). Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr, 2(2), 78–88.
Levine. M., Rumsey, S.C., Daruwala, R., Park, J.B., Wang, Y. (1999). Criteria and recommendations for vitamin C intake. JAMA, 281(15), 1415–1423.
Levy, T.E. (2012). Primal Panacea, MedFox publishing LLC.
O’Connor, C.J., Wallace, R.G., Iwamoto, K., Taguchi, T., Sunamoto, J. (1985). Bile salt damage of egg phosphatidylcholine liposomes. Biochim Biophys Acta, 817, 95–102.
Otake, K., Imura, T., Sakai, H., Abe, M. (2001). Development of a new preparation method of liposomes using supercritical carbon dioxide. Langmuir, 17(13), 3898–3901.
Padayatty, S.J., Sun, H., Wang, Y., Riordan, H.D., Hewitt, S.M., Katz, A., Wesley, R.A., Levine, M. (2004). Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med, 140, 533–537.
Panagiotou, T., Fisher, R.J. (2013). Producing micron- and nano- size formulations for functional foods applications. Func Foods in Health and Disease, 3(7), 274-289.
Patel, H.M., Ryman, B.E. (1976). Oral administration of insulin by encapsulation within liposomes. FEBS Lett, 62, 60–63.
Prinz, W., Bortz, R., Bregin, B., Hersch, M. (1977). The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int J Vitam Nutr Res, 47(3), 248-257.
Riordan, N.H., Riordan, H.D., Casciari, J.P.(2000). Clinical and experimental experiences with vitamin C. J Orthomol Med, 15, 201-213.
Rogers, J. A., Anderson, K. E. (1998). The potential of liposomes in oral drug delivery. CRC Crit. Rev. Ther. Drug Carrier Syst, 15, 421–480.
Rowland, R.N., Woodley, J.F. (1980). The stability of liposomes in vitro to pH, bile salts, and pancreatic lipase. Biochim Biophys Acta, 620, 400–409.
Schorah, C.J., Downing, C., Piripitsi, A., Gallivan, L., Al-Hazaa, A.H., Sanderson, M.J., Bodenham A. (1996). Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr, 63,760-765.
Shaji, J., Patole, V. (2008) Protein and Peptide drug delivery: oral approaches. Indian J Pharm Sci, 70, 269-277.
Shaji, J., Patole, V. (2008). Protein and Peptide drug delivery: oral approaches. Indian J Pharm Sci, 70, 269-277.
Szoka, F. Jr., Papahadjopoulos, D. (1978). Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA, 75(9), 4194–4198.
Taira, M.C., Chiaramoni, N.S., Pecuch, K.M., Alonso-Romanowski, S. (2004).Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Deliv. 11, 123-128.
Torchilin, V.P. (2005). Recent advances with liposomes as pharmaceutical carriers nature reviews. Drug discovery, 4, 145-160.
Uhumwangho, M.U., Okor, R.S. (2005). Current trends in the production and biomedical applications of liposomes: a review. Pak J Pharm Sci, 4(1), 9–21.
Vislisel, J.M., Schafer, F.Q., Buettner, G.R. (2007). A Simple and Sensitive Assay for Ascorbate Using a Plate Reader. Anal Biochem, 365(1), 31–39.
Wang, Y., Mackenzie, B., Tsukaguchi, H., Weremowicz, S., Morton, C.C., Hediger, M.A. (2000). Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun, 267(2), 488–494.