.
History of dietary change
In the early 19th century, arctic explorers and whalers began trading with the Inuit, which brought substantial changes to the traditional indigenous diet by introducing pilot biscuits, flour, sugar, coffee, tobacco and salt (Fediuk, 2002). Subsequent to this in the 1930s, pioneering nutritional studies were done by the dentist Weston A. Price. Price travelled the world examining pre-contact indigenous peoples to understand the reason behind formation of dental caries. He observed that there was an almost complete lack of carious and or deformed teeth, congenital skeletal malformations and a high immunity to infectious disease among indigenous peoples from every continent with low or no contact (Price, 1939).
One of the groups Price studied were the Inuit in northern Alaska. The traditional Inuit diet was comprised of salmon, salmon eggs, entire body of seal, caribou with organ meats, dried sea kelp, berries, sorrel grass (i.e. Cochlearia) preserved in seal oil, and entire body of whale (Price, 1939). A particularly important feature of the Inuit diet is the relatively high level of vitamin A and D, which is present in the liver organ meats, seal oil, whale blubber and fish eyes and eggs, as well as the moderate vitamin C levels that are found in the liver, adrenal glands of caribou, the blubber and skin of whales, the liver, brain and fat of seals (Iwama et al., 2012), (Fediuk, 2002), berries and sorrel grass also colloquially known as scurvy grass (Pereira, 1854). Additionally, the macronutrient ratios (i.e., protein ~56%, fat 43%, carbohydrate 1%) of the Inuit diet indicate that pre-contact Inuit people were in ketosis due to their very low carbohydrate intake (Heller & Scott, 1967). The carious teeth study reported by Price performed with the residents of Bethel, Alaska, on the Kuskokwim river is shown in Table 1. Types of diet clearly affected the proportions of dental caries.
Table 1. Comparisons of diet type with respect to proportion of caries (Price, 1939)
| Diet type | # of People | # of Teeth | Carious teeth | % Caries |
| Indigenous diet | 27 | 796 | 1 | 0.1 |
| Mixed diet | 21 | 600 | 38 | 6.3 |
| Processed diet | 40 | 1094 | 252 | 23 |
| Total | 88 | 2490 | 291 | 11.6 |
The processed diet consisted of white flour, white sugar, canned goods, coffee and tea and is inherently lacking in any trace minerals or vitamins A, D and C. The link between diet type and dental caries found by Price in the 1930s are a stark contrast to those by physicians in 1975 in the Canadian arctic in which neonatal hypertyrosinemia secondary to ascorbic acid deficiency was found (Clow, Laberge & Scriver, 1975). However, two things that the Canadian physicians failed to realize were that (1) Inuit in northern Quebec were living on a diet similar to the Mixed diet and the Processed diets; and (2) the dietary history of the Inuit as studied by Price before these diets were introduced to them. Among the traditional pre-contact Inuit, there had also never been any history of epidemic vitamin deficiency diseases such as pellagra or beri-beri (Draper, 1977; Sandler, 1951). The traditional Inuit diet was further changed by the Canadian Federal Family Allowance payments beginning in 1945, which were paid to all Inuit families with children under the age of 16 as trade goods (Wachowich, 1999). With the substantially increased transition into processed carbohydrate-rich food and concurrent reduction of traditional foods by ~33% lower of total intake (Kuhnlein, Soueida & Receveur, 1995), the need arose to fortify these foods so as to prevent scurvy and other nutritional deficiency diseases.
Traditional dietary remnants
Although completely reverting back to primitive indigenous diets today would be highly impractical in the modern world, remnants of the traditional indigenous diets do exist in many cultures globally. For instance, Asian and European cuisines all contain a wide variety of organ meats, fish and high fiber vegetables as part of their traditional cuisines (Table 2). What these cuisines have in common is minimal processing, higher fiber and low carbohydrate.
Table 2. Different Traditional Dishes
Cosentino, 2017; Child, Beck & Bertholle, 1961; List of Chinese Dishes, 2020, Wikipedia.
| Dish | Translation | Country of Origin |
| Haggis | Sheep stomach stuffed with offal and barley | Scotland |
| Liverwurst | Liver sausage | Germany |
| Sauerkraut | Pickled cabbage | Germany |
| Pate | Liver pudding | France |
| Aspic | Cold molded meat stock jelly | France |
| Moules | Mussels | France |
| Sofrit | Liver, kidney, heart & lung with onion & tomato | Italy |
| Hatsu/Gyutan Yakitori | BBQ chicken heart/Cow tongue | Japan |
| Tsukemono | Pickled vegetables | Japan |
| Sashimi | Raw fish | Japan |
| Pho tai sac | Beef and tripe noodle soup | Vietnam |
| Xue doufu | Blood curd | China |
| Xielian yudu | Crab & fish stomachs | China |
| Haishen | Sea cucumber | China |
| Fuchi feipian | Thin sliced beef and beef offal | China |
Adopting the traditional cuisines into modern diets would be beneficial due to (1) consuming entire animals thereby producing less wastage as well as lowering the ecological footprint of the diet, and (2) enhancing nutrition uptake since the mineral, amino acid profile and vitamin content of these foods is superior to modern processed diets (Scheid, Bennett & Schweigert, 1953), (Schweigert, Bennett & Guthneck, 1954) (Jodral-Segado et al., 2003).
In recent years there have been two growing dietary movements namely plant based diets and carnivore diets, which are at polar opposites to each other. Both of the extreme dietary movements have become politicized which has resulted in having inadvertently omitted the two key tenants of nutrition. The first key tenant being the absorption threshold of protein, which is ~0.53 g protein N kg-1 body weight (BW) in a healthy individual (Rudman et al., 1973). In high protein diets, there exists what is referred to as the protein ceiling, beyond which the liver cannot up-regulate enzymes necessary for urea synthesis (Rudman et al., 1973). Exceeding this threshold value known as the maximum rate of urea synthesis (MRUS) in normal healthy individuals of ~65 mg urea N h-1 kg-1 BW results in hyperammonemia, hyperaminoacidemia and encephalopathy (Rudman et al., 1973). In hunter-gatherer diets of people residing above 40° latitude, where dietary carbohydrate is increasingly scarce, excessive consumption of lean meats from wild game animals would result in excess MRUS and lead to the syndrome known colloquially as “rabbit starvation” (Cordain et al., 2000). Excess MRUS accounted for why the Inuit chose the fattiest parts of the animals they survived on in order to prevent “rabbit starvation” and leaving the leanest meats such as muscle for their dogs (Fediuk, 2002). The second tenant of diet involves the excess consumption of carbohydrate. A higher consumption of carbohydrate leads to problems of diabetes and heart disease that can ensue in modern industrialized diets where too much emphasis is placed on palatability (Sharma, Fernandes & Fulton, 2013).
Vitamin C transport in vivo
In the human body, vitamin C has two main forms; ascorbic acid (AA), and dehydroascorbate (DHA) (Packer, 1997). Vitamin C is transported into cells via several different transporters, where AA is taken up by the sodium-dependent vitamin C transporters SVCT1 and SVCT2 (Rivas et al., 2008). The SVCT1 is present mainly in epithelial tissues including kidney, liver, ovary, prostate, small intestine, colon, thymus, lung and pancreas, whereas the SVCT2 is more widely distributed in tissues including brain, retina, placenta, spleen, prostate, testis, ovaries, lung, skeletal muscle, intestine, kidney, adrenals and bone (Rivas et al., 2008). While DHA is taken up by the glucose transporters including GLUT1, GLUT3 and GLUT4, solely the GLUT1 transporter is used in AA recycling in humans (Hornung, & Biesalski, 2019).
One important way AA is recycled in the body is by uptake in its DHA form in the circulation by red blood cells (RBC). RBCs only express GLUT1 transporters in humans and not the SVCT family of transporters. When DHA is taken up, it is immediately reduced to AA inside the RBC via glutaredoxin and reduced glutathione (Padayatty & Levine, 2016). AA is then used both for structural integrity of the RBC, and as an electron pool that can be pumped out of the cell and into the plasma for further recycling of DHA to AA (Figure 1).
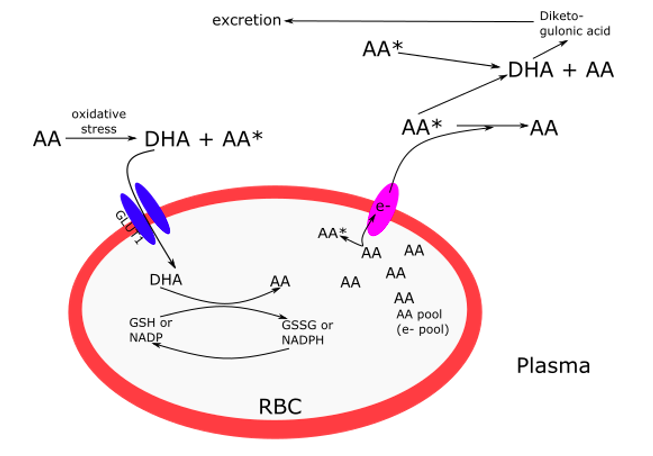
Figure 1. Ascorbic acid (AA) recycling in red blood cells (RBC) from dehydroascorbate (DHA) via the GLUT1 transporter and reduced glutathione (GSH). The larger the electron pool, the less DHA is produced, resulting in less excretion of diketogulonic acid. Adapted from figures from Padayatty & Levine, 2016; and Hornung & Biesalski, 2019.
Interestingly, in type 2 diabetics, both plasma and RBC levels of vitamin C are lower than in healthy individuals (Tu et al., 2015). High blood glucose levels seen in diabetic hyperglycemia, compete with DHA for absorption in humans through the GLUT1 transporter resulting in decreased RBC membrane deformability. This is due to lowered b-spectrin levels which are a protein that is part of the RBC structural skeleton (Tu et al., 2015). The deformability of RBCs are vital to microvascular flow and tissue oxygen delivery, which is compromised in type 2 diabetics and can result in retinopathy and amputations (Pippitt, Li & Gurgle, 2016). Another observation made by Willis & Fishman (1955) was of the acute deficiency of AA in arterial ground substance when atherosclerotic plaques were observed. The lack of AA causes a depolymerisation of the ground substance and subsequent plaque buildup in many chronic diseases such as cancer and diabetes, which further compromises microvascular blood flow (Willis, 1957). This is readily reversible with intravenous AA therapy and timelines for resolution are dependent on severity of plaques (Lamas et al., 2013; Willis, 1957).
One possible disadvantage of the conventional dietary advice from organizations such as the Heart and Stroke Foundation of Canada of “eating 7-10 servings of fruit or vegetables per day” is the accompanying increase in glycemic load, and the simultaneous competition of that glucose with DHA for ascorbate recycling (Tu et al., 2015). Indeed, the evolutionary advantage associated with losing the L-gulono-lactone-oxidase (GLO) enzyme responsible for producing AA revolves around frugality and AA seasonal scarcity (Hornung & Biesalski, 2019). It is not coincidental that the average lifespan of RBCs of 120 days corresponds to the frank expression of scurvy as AA rate of recycling in RBCs gradually declines until the critical plasma level of <10 mmol L-1 AA is reached (Carr & Cook, 2018; Léger, 2008).
Many different transporter proteins in cells can be up or down-regulated when there is either too much or not enough stimulation. An example of this is in addiction, where opiate receptors are inactivated when too much drug is present (Meyer & Quenzer, 2005). It is reasonable to hypothesize that several millennia of a lack of exposure to high carbohydrate loads would cause either an increase in the sensitivity of GLUT1 in the Inuit, or down-regulate it’s expression. This sensitivity to carbohydrate would seem to parallel that associated with alcohol in the Inuit and would afford an explanation for the lack of scurvy in the Inuit pre-contact with westerners, while explaining their increased susceptibility to diabetes, alcoholism and scurvy post-contact as described by Price.
Physiological areas of ascorbic acid concentration
The need for AA in different tissues of the body depends on its use as either an enzyme co-factor, or in areas where there is higher amounts of oxidative stress. Two important examples of the use of AA as an enzyme co-factor are in the enzyme, Dopamine-b-hydroxylase in the synthesis of catecholamines in the brain and adrenal glands, and in collagen hydroxylation with prolyl-4-hydroxylase (Padayatty & Levine, 2016). As previously mentioned in the history section, one possible benefit of dietary consumption of animal adrenal glands and brains by the traditional Inuit diet would be due to both higher contents of fat and vitamin C in these tissues (Fediuk, 2002).
Vitamin C is considered to be one of the most potent water-soluble antioxidants in the body in terms of its concentration and protective effect in prevention of plasma lipid peroxidation (Frei, England & Ames., 1989). In most mammals that synthesize their own AA, the highest concentrations of vitamin C in descending order exist in the adrenals, pituitary, brain, liver, lung and thyroid. Whereas in humans, the order is lymphocytes, monocytes, pituitary, adrenals, eye lens and brain, with the spleen, liver and pancreas having a similar level (Padayatty & Levine, 2016). Although AA concentration is different between synthesizing and non synthesizing species, it is yet another example of why the Inuit would choose the AA concentrated areas as the essential parts of the animal for consumption.
Another area of AA use relevant to glucose metabolism is in insulin secretion. Although the mechanism has not yet been elucidated, it appears that in scorbutic guinea pigs, AA is required in order for insulin to be secreted from pancreatic islets (Wells et al., 1995). The implications of AA requirement for insulin release are that in diets with elevated levels of carbohydrate and subsequent blood glucose, a decline in AA will make insulin secretion less robust, which will further potentiate progress towards metabolic syndrome and diabetes, although more studies will need to be done to determine if this is indeed the case.
Future research directions
Due to the molecular similarity between AA and glucose, requirements in individuals will vary based on the glycemic load of diet due to AA competition with glucose. This is illustrated by examination of the contents of traditional indigenous diets and their lack of scurvy and other chronic deficiency disease development. Human nutrition studies can either be classified as controlled, or free-living (Burri & Jacob, 1997). However, both systems have their disadvantages as complete control of human diet is impossibly complex. One possible way forward in determining ascorbate need in different clinical populations might be comparing data from type 2 diabetic populations compared to non-diabetics whom were being treated with intravenous AA. In fact, the Trial to Assess Chelation Therapy (TACT-1) did just this. In this ground breaking trial, the overall reduction in end points (MI, CVA and death) was reduced by ~18% in the general population, but when the subset of diabetics was analyzed there was an overall risk reduction of 45% (Lamas et al., 2013). Could this have been due to correction of a scorbutic micro-environment in diabetic vasculature? These clinical outcomes could, although indirectly, be tied to ascorbate kinetics and efficacy of treatment. It also underlines the need for lower levels of carbohydrate and higher levels of fat in diet in order to fully realize the wisdom of the Inuit and other indigenous diets.
References
Burri JB & Jacob RA (1997) Human metabolism and requirement for Vitamin C. In Vitamin C in health and disease. (pp. 341-366). New York, USA: Marcel Dekker Inc.
Carr AC & Cook J (2018) Intravenous Vitamin C for cancer therapy – Identifying the current gaps in our knowledge. Frontiers in physiology. 9: 1182. https://doi.org/10.3389/fphys.2018.01182
Child J, et al. (1961) Mastering the Art of French Cooking. Alfred A Knopf, New York.
Clow CL, Laberge C & Scriver CR (1975) Neonatal hypertyrosinemia and evidence for deficiency of ascorbic acid in Arctic and subarctic peoples. Canadian Medical Association journal. 113(7): 624–626.
Cordain L, Miller JB, Eaton SB, Mann N, Holt SH & Speth JD (2000) Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. The American journal of clinical nutrition. 71(3): 682–692. https://doi.org/10.1093/ajcn/71.3.682
Cosentino C (2017) Offal Good, cooking from the heart, with guts., Clarkson Potter Publishers, New York.
Fediuk K (2002) Vitamin C in the Inuit diet : past and present. Masters Thesis.
Frei B, England L & Ames BN (1989) Ascorbate is an outstanding antioxidant in human blood plasma. Proceedings of the National Academy of Sciences of the United States of America. 86(16): 6377–6381. https://doi.org/10.1073/pnas.86.16.6377
Heller CA, Scott EM (1967) The Alaska Dietary Survey 1956-61. Washington: U.S. Dept. of Health, Education and Welfare.
Hornung TC & Biesalski HK (2019) Glut-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis: The electron transfer hypothesis. Evolution, medicine, and public health. 2019(1): 221–231. https://doi.org/10.1093/emph/eoz024
Iwama M, Amano A, Shimokado K, Maruyama N & Ishigami A (2012) Ascorbic acid levels in various tissues, plasma and urine of mice during aging. Journal of nutritional science and vitaminology. 58(3): 169–174. https://doi.org/10.3177/jnsv.58.169
Jodral-Segado AM, Navarro-Alarcón M, de la Serrana HLG & López-Martı́nez MC (2003) Magnesium and calcium contents in foods from SE Spain: influencing factors and estimation of daily dietary intakes. Science of the total environment. 312(1-3): 47-58.
Kuhnlein HV, Soueida R & Receveur O (1995) Baffin Inuit food use by age, gender and season. Journal of the Canadian Dietetic Association.
Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J, Lee KL & TACT Investigators (2013) Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 309(12): 1241–1250. https://doi.org/10.1001/jama.2013.2107
Léger D (2008) Scurvy: reemergence of nutritional deficiencies. Canadian family physician Medecin de famille canadien. 54(10): 1403–1406.
List of Chinese Dishes (retrieved on May 10, 2020). https://en.wikipedia.org/wiki/List_of_Chinese_dishes
Meyer JS & Quenzer LF (2005) Psychopharmacology: Drugs, the brain, and behavior. Sinauer Associates.
Packer L (1997) Vitamin C in health and disease. 4. CRC Press.
Padayatty SJ & Levine M (2016) Vitamin C: the known and the unknown and Goldilocks. Oral diseases. 22(6): 463–493. https://doi.org/10.1111/odi.12446
Pereira J (1854) The elements of materia medica and therapeutics (Vol. 2). Blanchard and Lea.
Price WA (1939) Nutrition and Physical Degeneration 8th Ed., Price-Pottenger Publishing, CA
Rivas CI, Zúñiga FA, Salas-Burgos A, Mardones L, Ormazabal V & Vera JC (2008) Vitamin C transporters. Journal of physiology and biochemistry. 64(4): 357–375. https://doi.org/10.1007/BF03174092
Rudman D, DiFulco TJ, Galambos JT, Smith RB 3rd, Salam AA & Warren WD (1973) Maximal rates of excretion and synthesis of urea in normal and cirrhotic subjects. The Journal of clinical investigation. 52(9): 2241–2249. https://doi.org/10.1172/JCI107410
Sandler BP (1951) Diet prevents polio. Lee Foundation for Nutritional Research.
Scheid HE, Bennett BA & Schweigert BS (1953) Thiamine, riboflavin, and niacin content of organ meats. Food Research. 18: 109-112.
Schweigert BS, Bennett BA & Guthneck BT (1954) Amino acid composition of organ meats. Food Research. 19: 219-223.
Sharma S, Fernandes MF & Fulton S (2013) Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. International journal of obesity (2005). 37(9): 1183–1191. https://doi.org/10.1038/ijo.2012.197
Tu H, Li H, Wang Y, Niyyati M, Wang Y, Leshin J & Levine M (2015) Low red blood cell Vitamin C concentrations induce red blood cell fragility: A link to diabetes via glucose, glucose transporters, and dehydroascorbic acid. EBioMedicine. 2(11): 1735–1750. https://doi.org/10.1016
Pippitt K, Li M & Gurgle HE (2016) Diabetes Mellitus: Screening and Diagnosis. American family physician. 93(2): 103–109.
Wachowich NS (1999) Stories from the lives of three Inuit women. Montreal & Kingston:McGill-Queen’s University Press.
Wells WW, Dou CZ, Dybas LN, Jung CH, Kalbach HL & Xu DP (1995) Ascorbic acid is essential for the release of insulin from scorbutic guinea pig pancreatic islets. Proceedings of the National Academy of Sciences of the United States of America. 92(25): 11869–11873. https://doi.org/10.1073
Willis GC & Fishman S (1955) Ascorbic acid content of human arterial tissue. Canadian Medical Association journal. 72(7): 500–503.
Willis GC (1957) The reversibility of atherosclerosis. Canadian Medical Association journal. 77(2):106–108.