Introduction
Schizophrenia and associated psychotic disorders affect approximately 0.7% of the population over their lifetime (van Os, Kenis & Rutten, 2010). While the exact pathogenesis of these conditions remains unclear, a variety of factors including genetic predisposition, neurotransmitter imbalance and social factors are known to be involved. There are several domains of symptoms including positive symptoms such as delusions and hallucinations, negative symptoms such as withdrawal and low motivation as well as depression, anxiety and cognitive symptoms (American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM- 5®). American Psychiatric Pub, 2013). In addition to schizophrenia’s impact on functioning, emotional health and quality of life, there is also a highly significant increase in physical illnesses within this population including high rates of obesity, cardiovascular disease and diabetes resulting in a life-expectancy that is estimated at 8 to 10 years shorter than the general population (Gatov, Rosella, Chiu, & Kurdyak, 2017). While anti-psychotic medications are known to contribute to the high rates of weight gain and metabolic syndrome, it is established that derangement of metabolic pathways exists in anti-psychotic naïve patients and thus appears to be related to the illness pathology as well (Mehrul, 2016).
With so little known about the exact pathogenesis, and wide clinical heterogeneity along the schizophrenia spectrum, many avenues have been explored in search of increased understanding of the disease and therapeutic options.
Interest in the role of vitamins in psychosis dates back 50 years. Many mechanisms have been proposed for the role of vitamins including the role of deficiency, the potential for increased need in certain populations and modulation of various biochemical pathways (Hoffer & Prousky, 2008).
One pathway that has been proposed recently to be involved in psychosis is the one-carbon metabolism cycle. This pathway is important in the creation of the precursors to DNA as well as the methylation of membrane lipids and DNA. Methyl groups are transferred between a number of molecules including homocysteine, S-adenosyl-Methionine (SAMe) and folic acid. The pathway depends on the availability of various cofactors including the active forms of vitamin B12, vitamin B6 and folic acid (Frankenburg, 2007).
Vitamin B12 (cobalamin) is a water-soluble nutrient. There are several causes of B12 deficiency. The primary source of dietary B12 is meat which may not be consumed due to personal choice or financial limitations. Absorption of dietary B12 relies on a complex process and may be hindered by in adequate pancreatic enzymes, lack of intrinsic factor, gastrointestinal disorders and medications. Symptoms of B12 deficiency include fatigue and neurological complaints such as altered sensation, loss of coordination and weakness; however, many people with low levels appear asymptomatic (Frankenburg, 2007). There is controversy around the ideal blood levels of B12 and as such the exact definition of deficiency or insufficiency status is unclear (Frankenburg, 2007). B12 deficiency is also known to cause nerve degeneration and demyelination which may impact neurotransmission (Shils & Shike, 2006). Vitamin B12 exists in several forms, the most important being methylcobalamin which participates in the activation of folic acid and the one-carbon metabolism cycle. Other forms, including cyanocobalamin, a common form used in vitamin B12 supplements, must be metabolized to the active form in order to be biologically active (Shils & Shike, 2006).
Folic acid is found in the human diet in a variety of foods including leafy green vegetables; however, it is sensitive to heat and can be destroyed during cooking. Various medications also impact the absorption and storage of folic acid leading to deficiency. Like vitamin B12, folic acid exists in various forms. Folic acid is the stable, fully oxidized form of folate. Conversion to the active form methyltetrahyrofolate (MTHF) is required for metabolic activity (Shils & Shike, 2006). Folinic acid is a medication used to treat the side effects of folic acid reducing medications; it is a modified version of folic acid that is converted to the active form within the body (Scaglione & Panzavolta, 2014). The relevance of folic acid in psychotic disorders may have to do with a genetic polymorphism, genetic variations that affect function. The enzyme methyltetrahyrofolate reductase (MTHFR) is coded on chromosome 1 and converts folic acid to the active form, MTHF which participates in the one-carbon metabolism cycle. Individuals with genetic polymorphisms have reduced activity of this enzyme and subsequently have increase blood homocysteine levels. The occurrence of the polymorphisms has been associated with cardiovascular disease, stroke, congenital abnormalities, increase risk of cancer and more recently with psychiatric disorders including schizophrenia; however, there are studies with conflicting results (Frankenburg, 2007).
This paper will review the current evidence, as obtained through a systematic literature search, for the role of these vitamins in the pathogenesis and treatment of psychotic disorders as well as reviewing possible mechanisms and providing recommendations for further research and clinicians.
Methods
The present review is a sub-analysis of a larger scoping review on the topic of dietary factors in the development and progression of psychotic disorders. The scoping review featured an a priori search strategy developed by an experience medical information specialist which included over 300 search terms. The search was executed in Embase, Embase Classic and Ovid MEDLINE on January 6, 2017 and updated on April 15 2018. Eligible studies included original research on dietary patterns or dietary constituents in patients with psychotic disorders or animal models of psychosis including clinical trials, observational studies, case reports and meta-analyses. Studies were required to assess a mental health outcome and not just side effects of medication or physical health outcomes. Title and abstract screening were complete in duplicate by two reviewers using Abstrackr, an online open-source program that facilitates rapid screening decisions and concurrent tagging of results. Disagreement was resolved by consensus by the two principle investigators. Studies that failed to meet inclusion criteria on full text review were excluded. Data was extracted using a template and studies were organized by methodology for analysis.
.
Results
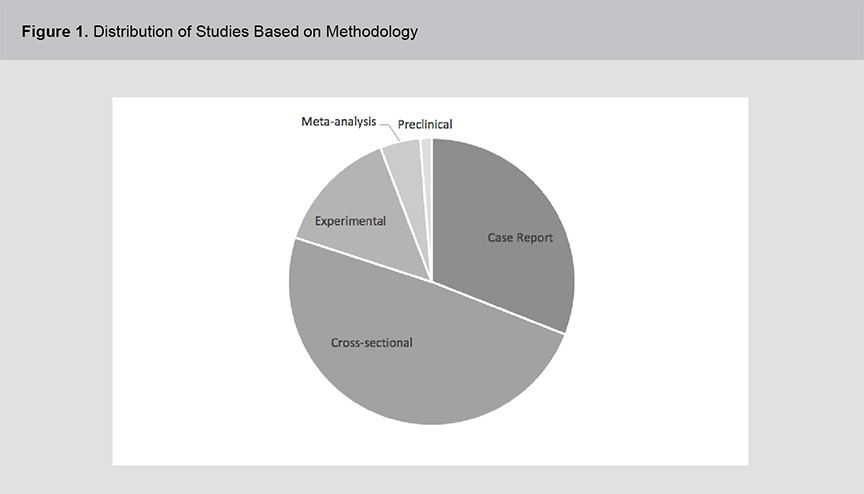
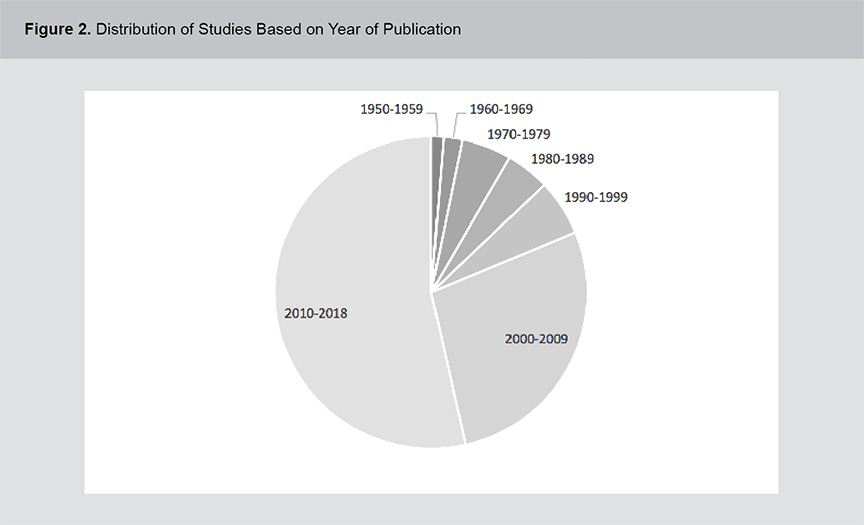
A significant body of evidence exists related to the role of vitamins B12, B6 and folic acid in psychosis pathogenesis and treatment including a range of study methodologies. Figures 1 and 2 show the distribution of studies based on methodology and year of publication. The studies were primarily cross-sectional in design and although the field of study dates back to the 1950s, the volume of literature has expanded rapidly in the past 2 decades. For a full list of the articles included in this review, please see appendix 1.
Cross-Sectional Evidence
Folate: Thirty-four studies found decreased levels of folic acid in patients with psychotic disorders and four found decreased intake of dietary folate. Nineteen showed no difference when compared to controls or reported that folic acid levels we within normal limits. Of these studies, 14 assessed individuals with FEP, 42 assessed participants with chronic illness and one assessed a combination. One found decreased levels of the active form of folic acid in cerebrospinal fluid (Ramaekers, 2014). The remaining studies assessed folate levels in either the serum, plasma or red blood cells. Several studies reported associations between lower levels of folate and higher severity of negative symptoms (Chen 2014; Song, 2014, Goff, 2004, Tuga, 2011), total psychopathology (Herrán, 1999, Song, 2014), depression (Lerner, 2006) and disorganization (Herrán, 1999). On study found reduced levels of folate only in participants with the MTHFR polymorphism (Roffman 2008). Four meta-analyses have been completed since 2016 including 2000 to 3700 participants, including separate analyses of FEP and chronic schizophrenia. All analyses found lower levels of folic acid in these populations compared to healthy controls.
Vitamin B12: A number of studies have compared the levels of blood B12 in patients with psychotic disorders to healthy controls or other patient groups. Twenty studies reported decreased levels in the individuals with psychosis. One study found an increased level of B12 in these participants with psychosis and 23 studies reported no difference between participants with psychosis and controls. Two meta-analyses published in 2016 and 2017 including the results of more than 2000 participants reported no difference in B12 levels in cases compared to controls.
Vitamin B6: Eleven cross-sectional studies have compared blood levels of vitamin B6 in participants with primarily chronic psychotic disorders to control subjects. Nine reported decreased levels and two reported no association. A recent meta-analysis analyzing a total of 2125 patients reported lower levels of serum B6 in participants with chronic schizophrenia compared to healthy controls (Tomioka, 2018).
Case Reports
Folate: Six case reports have documented low levels of folate in patients experiencing a first episode of psychosis and one in a patient with chronic illness. Six of these reported implementing supplementatal folic acid and five reported significant improvement or resolution of symptoms; one reported no change with folinic acid supplementation but did not follow the patient when they discontinued this treatment and began supplementation with MTHF (Ho, 2010).
Vitamin B12: Thirty-six case reports have documented low levels of vitamin B12 in patients with psychotic disorders. These were primarily in individual first episode cases although one reported on 19 multi-episode cases. Thirty-seven case reports document improvement or resolution of psychosis symptoms in response to B12 supplementation.
Vitamin B6: Four case reports, two in FEP, two in chronic psychosis, reported the results of vitamin B6 supplementation. Doses ranged from 100mg to 500mg/day and all studies reported benefit, including one report of resolution of symptoms (Brooks, 1983)
Pre-Clinical Studies
Two studies have used folic acid supplementation in animal models of psychosis. One reported improvement in positive symptoms while the other reported improvement in negative symptoms and no change in positive symptoms. Both reported decreased levels of oxidative stress in brain tissue and subsequently, less damage to brain tissues.
Experimental Studies
Folic Acid: Seven experimental trials studied the effect of folic acid supplementation in individuals with chronic psychosis. Six of these reported a benefit in at least one symptom domain. One reported improvement in cognitive symptoms, three in overall symptoms. Two studies reported improvement in negative symptoms while two reported no change. One study showed an improvement in positive symptoms. Most of the studies used the folic acid as an adjunctive therapy in addition to medication and the doses varied quite dramatically, from 0.5mg to 90mg per day.
Vitamin B12: Two experimental studies from 1955 were located which used supplemental vitamin B12 in individuals with chronic psychosis; neither reported an effect.
Folic Acid and Vitamin B12: Three randomized, controlled trials used a combination of vitamin B12 and folic acid as adjunctive therapies in participants with chronic schizophrenia. The studies were 12-16 weeks in duration involving 22, 42 and 140 participants. All reported positive outcomes including total psychosis symptoms, negative symptoms and biological markers including cortical thickness and fMRI measurements associated with cognitive function.
Vitamin B6: Ten experimental studies have used supplemental vitamin B6 in patient with chronic psychosis. Four combined the vitamin with other interventions including zinc (one study), L-tryptophan (1 study) and the medication L-Dopa (2 studies) and all used the supplement as an adjunctive therapy in addition to medication. Nine studies reported some level of improvement in at least one symptom domain including total psychopathology, negative symptoms, depression and anxiety symptoms and EEG results. The studies were small with 8 to 40 participants and four utilized randomization, placebo control and blinding.
All: A meta-analysis combined the results of randomized controlled clinical trials using adjunctive vitamin and mineral supplements in patients with schizophrenia. When they pooled the results of 7 studies assessing vitamins B12, B6 and folic acid, a statistically significant benefit over placebo was observed for total psychopathology symptoms but not for positive or negative symptoms individually (Firth 2017).
Discussion
While mixed and limited in methodological quality, there is evidence that vitamins B12, B6 and folic acid may be relevant in the development and progression of psychotic disorders. The mechanism by which these vitamins may contribute to improvements in psychosis are not known but hypotheses exist.
One theory has to do with the one-carbon metabolism cycle. Homocyestine is a sulfur-containing amino acid that is part of the cycle. It is converted to methionine in a reaction that requires vitamin B6. In addition to known associations between hyperhomocysteinemia and cardiovascular disease, elevated levels have also been reported in patients with schizophrenia, with correlations found between higher levels of homocycteine and worse psychopathology (Misiak, 2014). The molecule is thought to exert neurotoxic effects, possibly through stimulation of the N-methyl-D-aspartate receptor (Frankenburg, 2007). Homocysteine exerts its detrimental cardiovascular effect through an increase in oxidative stress (Tyagi, Sedoris, Steed, Ovechkin, Moshal, & Tygi, 2005). There is a growing body of evidence connecting elevated brain levels of oxidative stress with the pathophysiology of schizophrenia include high levels in post-mortem brains, elevated SOD and reduced glutathione in the serum of FEP patients, a variety of pre-clinical data, as well as proposed mechanisms involving damage to parvalbumin interneurons and myelin (Emiliani, Sedlak, & Sawa, 2014). In addition to vitamin B6, B12 and folic acid also play roles in this metabolic pathway and deficiency of any of these vitamins can lead to elevation in homocysteine.
In addition to the role in one-carbon metabolism, vitamin B12 and folic acid play a role as methyl donors for many methylation reactions that take place within the brain. A hypothesis exists that hypomethylation impacts neurotransmitter synthesis and consequently, psychiatric illness (Milanlıoğlu, 2011). Vitamin B6 also serves as a cofactor in neurotransmitter synthesis including serotonin and dopamine (Skarupski, Tangney, Li, Ouyang, Evans, & Morris, 2010).
Deficiencies of any of these vitamins may play a role in the development of psychopathology. Additionally, variations in metabolism such as genetic polymorphisms affecting the activation of folic acid may be involved. The impact of this reduced enzymatic function seems to be related to a few factors. The process of converting folic acid (found in food and many supplements) to L-methylfolate allows for direct penetration of the blood brain barrier (Wu & Pardridge, 1999). A study in patients with cardiovascular disease found that supplementing with MTHF resulted in bioavailability seven-fold higher than folic acid (Willems, Boers, Blom, Aengevaeren, & Verheugt, 2004). Of the studies included in this review, two utilized a supplement containing the active form; both reported benefit.
The heterogeneity of the data, including studies showing benefit or no effect, may be related to some of the above mechanisms. A number of case reports have reported states of deficiency and significant benefit from correcting these deficiencies. Observational data assessing for deficiencies in patients with psychosis shows a mixture of results – some report deficiency, some report no association. It is possible that deficiency of the studied vitamins is present in a subpopulation of patients with psychotic disorders and that when analyses look at average levels within a sample of patients, the combined results may not reach statistical significance. Likewise, experimental studies that provide vitamin supplements to a heterogeneous group of patients with psychosis may fail to detect benefit among subpopulations who are deficient or have unique factors such as deficiencies states and genetic polymorphisms or other variations in metabolism making them potentially more responsive to these interventions.
Further research in the area of vitamins and psychosis is warranted. Studies may benefit from testing patients for deficiency status or genetic polymorphisms in order to better elucidate the populations most likely to benefit from these interventions.
Application to the Clinical Management of Patients with Psychotic Disorders
Clinicians may consider testing patients affected by psychotic disorders for deficiencies in vitamin B12 and folic acid or measuring homocysteine as a functional marker for adequacy of vitamins B12, B6 and folic acid. Additionally, genetic testing can assess for polymorphisms in the MTHFR gene which would indicate a need to supplementation with the active form of folic acid.
Based on the results of testing or other clinical signs and symptoms, clinicians should consider recommending a supplement of one or more of these nutrients as an adjunctive therapeutic agent. Doses used in experimental studies showing benefit were 400ug of vitamin B12, 2-15mg of folic acid and 30-150mg of vitamin B6. Because of the need to activate certain supplemental forms of these nutrients prior to metabolic activity, clinicians may consider supplementing with the active forms of the nutrients: methyltetrahyrofolate, methylcobalamin and pyridoxal-5-phosphate. As with any intervention, the financial cost of testing or supplements should be considered and the patient monitored for adverse events, although none were reported in the studies summarized above.
Conclusions
The exact role of vitamins B12, B6 and folic acid in the pathogenesis and treatment of psychotic disorders is not fully understood; however, there is significant evidence to suggest that there is an association. Attention to the nutritional status of patients with psychosis is warranted, as is further research in the form of intervention studies, especially those that identify sub-populations most likely to benefit.
Disclosure Statements
The authors do not have any conflicts of interest.
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub.
Emiliani, F. E., Sedlak, T. W., & Sawa, A. (2014). Oxidative stress and schizophrenia: recent breakthroughs from an old story. Current Opinion in Psychiatry, 27(3), 185.
Frankenburg, F. R. (2007). The role of one-carbon metabolism in schizophrenia and depression. Harvard Review of Psychiatry, 15(4), 146-160.
Gatov, E., Rosella, L., Chiu, M., & Kurdyak, P. A. (2017). Trends in standardized mortality among individuals with schizophrenia, 1993–2012: a population-based, repeated cross-sectional study. Canadian Medical Association Journal, 189(37), E1177-E1187.
Hasnain, M. (2016). Schizophrenia and metabolic dysregulation: shared roots? The Lancet Psychiatry, 3(11), 1003- 1005.
Hoffer, A., & Prousky, J. (2008). Successful treatment of schizophrenia requires optimal daily doses of vitamin [B. sub. 3]. Alternative Medicine Review, 13(4), 287-292.
Milanlıoğlu, A. (2011). Vitamin B12 deficiency and depression. Journal of Clinical and Experimental Investigations, 2(4).
Scaglione, F., & Panzavolta, G. (2014). Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica, 44(5), 480-488.
Shils, M. E., & Shike, M. (Eds.). (2006). Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins.
Skarupski, K. A., Tangney, C., Li, H., Ouyang, B., Evans, D. A., & Morris, M. C. (2010). Longitudinal association of vitamin B6, folate, and vitamin B12 with depressive symptoms among older adults over time. The American Journal of Clinical Nutrition, 92(2), 330-335.
Tyagi, N., Sedoris, K. C., Steed, M., Ovechkin, A. V., Moshal, K. S., & Tyagi, S. C. (2005). Mechanisms of homocysteine-induced oxidative stress. American Journal of Physiology-Heart and Circulatory Physiology, 289(6), H2649-H2656.
van Os, J., Kenis, G., & Rutten, B. P. (2010). The environment and schizophrenia. Nature, 468(7321), 203.
Willems, F. F., Boers, G. H., Blom, H. J., Aengevaeren, W. R., & Verheugt, F. W. (2004). Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease. British Journal of Pharmacology, 141(5), 825-830.
Wu, D., & Pardridge, W. M. (1999). Blood-brain barrier transport of reduced folic acid. Pharmaceutical Research, 16(3), 415-419.