List of Abbreviations:
CRP: C-Reactive Protein
ENT: Ear-Nose-Throat
FC: Faecal Calprotectin
FODMAP: Fermentable Oligosaccharides, Disaccharides, Monosaccharides And Polyols
GAPS: Gut And Psychology Syndrome/Gut And Physiology Syndrome
GIT: Gastro-Intestinal Tract
IBD: Inflammatory Bowel Disease
OCD: Obsessive-Compulsive Disorder
PMS: Premenstrual Syndrome
QOL: Quality Of Life
SCD: Specific Carbohydrate Diet
UC: Ulcerative Colitis
Introduction
Definition
Inflammatory bowel diseases (IBD), chiefly Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory diseases of the gastro-intestinal tract (GIT). The high level of inflammation manifests itself through recurrent ulcers (Pithadia & Jain, 2011) and leads to the activation of macrophages, formation of granulomas, and secretion of various cytokines such as TNF-α and interleukins (Papadakis & Targan, 2000, as cited by Pithadia & Jain, 2011).Transmural granulomatous inflammation (due to a benign nodule housing activated immune cells) in any part of the bowel is common in CD, while the inflammation displayed in UC tends to be limited to the colon’s mucosa (Tomasello et al., 2015). Colonoscopies in patients with CD reveal “lesions, cobblestoning, ulcerations and strictures” while colonoscopies in UC patients reveal “pseudopolyps and continuous areas of inflammation” (Mentella, Scaldaferri, Pizzoferrato, Gasbarrini & Miggiano, 2020, p.1).
The main symptoms of IBD are abdominal pain, diarrhoea, nausea, weight loss, anaemia, and blood in stool. Patients might also experience extraintestinal symptoms such as arthritis, sclerosing cholangitis, eye conditions (uveitis, iritis), and skin conditions (pyoderma gangrenosum, erythema nodosum) (Fiocchi, 1998, as cited by Pithadia & Jain, 2011).
Prevalence of CD and UC is increasing worldwide with estimated rates ranging from 25.9 to 318.5 cases per 100,000 persons (Mentella et al., 2020). In general, prevalence rates are significantly lower in Europe and Asia compared to the US (Burisch & Munkholm, 2015).
Costs induced by IBD’s diagnosis, assessment, treatment and supervision have been estimated to be around seven billion dollars (Dahlhamer, Zammitti, Ward, Wheaton & Croft, 2016) and are growing in parallel with the increasing prevalence. Patients who suffer from IBD score lower on quality of life (QOL) indicators. This is unsurprising given the comorbidities, relapses, frequent hospitalisations, and surgical procedures (Bewtra, Su & Lewis, 2007; Cohen, 2002).
Malnutrition
Malnutrition is common amongst IBD sufferers. Patients limit their food intake in order to avoid gastro-intestinal symptoms caused by numerous food intolerances (Lucendo & Rezende, 2009; Tomasello et al., 2015; Vagianos, Bector, McConnell & Bernstein, 2007).
Malnutrition is also caused by: (1) gastro-intestinal losses (diarrhoea, rectorrhagia, enteropathy, etc.); (2) poor absorption of nutrients; (3) metabolic disorders and (4) drug interactions. The following dietary deficiencies are commonly reported in IBD: iron, zinc, selenium, and vitamins A, B, C, D, and E (Lucendo & Rezende, 2009; Tomasello et al., 2015).
Aetiologies
The causes of IBD are still considered unclear (Maruszewska-Cheruiyot, Donskow-Łysoniewska, & Doligalska, 2018; Mentella et al., 2020). However, as is the case for most inflammatory diseases, IBD is believed to be multifactorial stemming from different factors: genetics, environment, and immunology (Pithadia & Jain, 2011).
Literature suggests a family history in up to 12% of IBD patients (Moller, Andersen, Wohlfahrt & Jess, 2015), creating an idea that there may be a hereditary component.
From an environmental perspective, there are multiple risk factors that come into play; “smoking, diet, psychological stress, non-steroidal anti-inflammatory drugs, and oral contraceptives, appendectomy, breastfeeding, as well as infections” all have a significant impact on the onset, development, and maintenance of IBD (Tomasello et al., 2015, p.81).
The “new feeding habits” are becoming a central factor when thinking of environmental aetiologies of IBD. Lucendo & Rezende (2009) use the term “new feeding habits” to refer to the “high consumption of sugar and refined carbohydrates” (p.2082).
Similarly, Tomasello et al. (2015), in their review on IBD and nutrition, identify the “Western diet” as a risk factor – that is, a diet characterised by an overabundance of refined sugar, processed carbs, processed omega-6 fats (sunflower oil, soy, corn, and margarines), food additives and heavy metals. In addition, Sakamato et al. (2005) found that higher consumption of sugar, sweeteners, carbohydrates, fats, and vegetable oils were positively associated with IBD rates. As mentioned by Mentella et al. (2020): “the greater incidence and prevalence of IBD in North America […] support a correlation between incidence of IBD and living according to a Western lifestyle” (p.2).
Western diet and environment have a direct impact on gut health and microbiota composition (Mentella et al., 2020; Pithadia and Jain, 2011; Sakamato et al., 2005; Tomasello et al., 2015; Yatsunenko et al., 2012). An altered gut microbiota composition, or gut dysbiosis, has been repeatedly found in IBD patients. Research suggests that IBD patients display an increased abundance of pathogenic flora, a decreased presence of beneficial flora, and an overall loss of microbiome biodiversity (Frank et al., 2007, 2011; Gevers et al., 2014; Li, Butcher, Mack & Stintzi, 2015; Morgan et al., 2012; Ott et al., 2004; Wong et al., 2022). Research identified a decrease in species responsible for butyrate production. Butyrate is a short chain fatty acid that positively modulates intestinal homeostasis and reduces inflammation (Christl, Eisner, Dusel, Kasper, & Scheppach, 1996; Looijer–Van Langen & Dieleman, 2009; Machiels et al., 2014; Mentella et al., 2020, p.4, citing Andoh, Bamba, & Sasaki, 1999). In addition, Lloyd-Price et al. (2019) found that functional dysbiosis was particularly observed during flares of IBD. In their elegant paper, Wu & Wu (2012) define IBD as an autoimmune disease caused by gut dysbiosis attacking the GIT. Other researchers have defined IBD as an autoimmunological disorder (Maruszewska-Cheruiyot, Donskow-Łysoniewska, & Doligalska, 2018)
Genetic analyses have also revealed that most of the genetic polymorphisms that predispose to IBD are in fact involved in host mucosal barrier function and host-microbiome interactions (Dheer et al., 2016; Elinav et al., 2011; Jostins et al., 2012; Lee & Mazmanian, 2010; McGovern et al., 2010, Rausch et al., 2011), suggesting that gut dysbiosis cannot be a simple consequence of IBD. Rather, it is likely to be a causative factor (Mentella et al., 2020).
Thus, even though the causes of IBD have been considered unknown, authors such as Lane, Zisman & Susking (2017) have recently concluded that genetically predisposed patients suffer from chronic intestinal inflammation due to aberrant mucosal immune response and an overall dysbiotic microbiome.
Gut Dysbiosis
Gut health and its impact on general health have been gaining the attention of researchers. The gut microbiome is impressively large. It is thought to contain between 500 and 1000 distinct bacterial species, fungi, and viruses (Qin et al., 2010). In addition, compared to the rest of the human body, it contains ten times more microorganisms and one hundred times more genes (Ley, Peterson, & Gordon, 2006). These microorganisms are responsible for vitamin production, hormone production, neurotransmitter production, repression of pathologic microorganisms, absorption and digestion of dietary substrates, and modulation of the immune system (Belkaid & Hand, 2014; Lane, Zisman & Susking, 2017; Lerner, Ramesh, & Matthias, 2019).
Our gut flora develop in utero and during early years of life. Factors such as mother’s gut flora, birth (natural or caesarean), or breastfeeding (or formula-feeding) are vital. Once host-microbiome homeostasis is reached in early childhood, the balance is supposed to remain stable, unless antibiotics, processed foods, agricultural chemicals and pharmaceuticals damage this homeostasis (Lozupone, Stombaugh, Gordon, Jansson, & Knight, 2012). In part due to the current Western lifestyle, many people in the industrialised world today suffer from gut dysbiosis (Campbell-McBride, 2020).
Gut dysbiosis (or abnormal gut flora) is responsible for abnormal cell regeneration, intestinal permeability (leaky gut), malabsorption of foods, allergies and intolerances, and production of toxic substances which are released into the bloodstream (Camilleri, 2019; Din & Alam, 2020; Fasano, 2011, 2012; Kaji et al., 1976; Lucendo & Rezende, 2009; Ngo, Neurath, & López-Posadas, 2022; Sapone et al., 2006; Sturgeon & Fasano, 2016; Tomasello et al., 2015; Zioudrou, Streaty, & Klee, 1979). Mentella et al. (2020) indicated that the “depletion of commensal microbes can result in impaired mucosal healing, chronic mucosal inflammation and colitis” (p.2). These are increasingly recognised as the central mechanisms to the understanding of IBD, food allergies, and malnutrition.
A Role for Glyphosate
Glyphosate is the active ingredient in the herbicide Roundup, and it is pervasive in the food supply in the United States. Recently, the U.S. Centers for Disease Control found that 80% of a random sample of people in the United States had detectable levels of glyphosate in their urine (United States Centers for Disease Control, June 2022). In 2014, Swanson, Leu, & Abrahamson published a paper which showed striking correlations between glyphosate usage on corn and soy crops in the United States and a long list of chronic diseases. One of the diseases they examined was inflammatory bowel disease. They included a plot that showed remarkable correlations between the rise in glyphosate usage and the rise in IBD over time (R = 0.94, p < 0.00000007).
While correlation does not necessarily mean causation, there is significant evidence from the research literature that provides plausible reasons for causality. First of all, glyphosate is patented as an antimicrobial agent, and it has been shown experimentally that many gut microbes are sensitive to glyphosate. Two of the most sensitive genera are Lactobacillus and Bifidobacteria, and these two are beneficial microbes that perform many important functions for the host (Shehata, Schrödl, Aldin, Hafez, & Krüger, 2013). Pathogens such as Clostridia are less sensitive, and the result is an overgrowth of pathogenic species and an inflammatory response due to immune cell infiltration to control the pathogens. Further support comes from a study published in 2017 which demonstrated experimentally that glyphosate disrupts the tight junctions of epithelial cells, causing a leaky gut (Gildea, Roberts & Bush, 2017).
Furthermore, glyphosate has been shown to disrupt the pH of the gut, raising the pH in the colon to the point where acid-loving microbes do not thrive. This results in a reduction in the supply of short chain fatty acids, particularly acetate and butyrate, to the gut colonocytes, whose primary nutrient source is butyrate. A controlled study involving exposing Sprague Dawley rats to differing amounts of glyphosate found a high correlation between glyphosate levels in the colon and the pH of the feces (R=0.72, p < 0.001). The pH increased linearly with increasing glyphosate levels in a dose-response relationship, ranging from a low of 5.0 to a high of 6.2. A corresponding decrease in acetate levels in the cecum was observed, from a high of 80 to a low of 40 mmol/kg (R=0.54, p< 0.0001) (Nielsen et al., 2018). A study specifically addressing the effects of pH changes in the human colon on the synthesis of butyrate by gut microbes found that the amount of butyrate in the colon was highly sensitive to pH. The concentration of butyrate was five times as high in an anaerobic culture maintained at pH 5.5 (25 mM) compared to pH 6.5 (5 mM) (Bedford & Gong, 2018).
Treatments
In their thorough review of existing treatments for IBD, Pithadia and Jain (2011) describe different categories of medications: steroids, aminosalicylates, corticosteroids, immunosuppressants, monoclonal antibodies, antibiotics, analgesics, antidiarrheals, sodium cromoglycate, or thalidomide. Enteral feeding is a common practice to treat malnutrition.
However, most existing treatments for IBD fail to reverse the underlying pathogenic mechanisms of acute inflammation in the gut (Grisham, 1994, as cited by Pithadia & Jain, 2011; Maruszewska-Cheruiyot, Donskow-Łysoniewska, & Doligalska, 2018) and some might even increase inflammation by disrupting gut homeostasis (Lozupone et al., 2012). Relapses are frequent (Mentella et al., 2020), suggesting that the root cause of the disease is not addressed by these treatments.
Alternative interventions focussing on rebalancing the gut flora by introducing probiotics have successfully decreased the inflammatory state. Wong et al. (2022) led a Lactobacillus casei strain Shirota (LcS) intervention on colitic mice. The gut flora balance was successfully re-established, and pro-inflammatory mediators interferon-gamma and nitric oxide were suppressed. The expression of the anti-inflammatory mediator interleukin-10 had increased. Bibiloni et al. (2005) administered probiotic treatment to 34 patients with active UC, who did not respond to conventional treatment. After six weeks with daily administration of 3600 billion bacteria per day, 53% displayed remission in sigmoidoscopies and another 24% demonstrated a positive response.
Butyrate has also been studied for its role in modulating the gut flora and anti-inflammatory effect (Bedford & Gong, 2018). Researchers found that butyrate successfully treats patients with UC (Lucenzo & Rezende, 2009, citing Assumpção, Rodrigues & Barbieri, 1999), which further suggests the importance of addressing gut microbiome composition. Faecal microbiota transplantation has been found to prompt remission in cases of active UC (Narula et al., 2017). Finally, helminth therapy is being increasingly studied as a potential treatment for IBD (Arai & Lopes, 2022; Maruszewska-Cheruiyot, Donskow-Łysoniewska, & Doligalska, 2018; Weinstock & Elliott, 2013). Helminths are parasitic worms which exert immunomodulatory functions by being able to inhibit or modify other ongoing immune responses (Arai & Lopes, 2022; Helmby, 2015; Moreels & Pelckmans, 2005; Weinstock & Elliott, 2013). To do so, they need to exist in balance with the rest of the microbial community. The western diet disrupts this balance and suppresses the growth of worms. In fact, helminth infections have practically been eradicated in high-income countries (Arai & Lopes, 2022; Helmby, 2015). While this might seem like a victory at first glance, it allows other pathogens to take over and cause disease. The eradication of worm infections has been accompanied by an explosion of chronic inflammatory conditions (Arai & Lopes, 2022; Helmby, 2015). It seems appropriate to quote Berilli, Di Cave, Cavallero, and D’Amelio (2012) here: “while in less-favoured areas, the interest in intestinal helminthiasis is mainly focused on the parasitic disease itself, in industrialised countries the intimate relationships between intestinal helminths with gut microbiota and the putative down-regulation of self-pathogenic immune response have been the object of recent studies” (p.2). These findings are in line with the hygiene hypothesis, suggesting that an overly clean environment prevents the correct development of the immune system (Helmby, 2015).
Helminth therapy consists of administering helminth eggs to the patient (either orally or percutaneously) that will hatch in the digestive system (Helmby, 2015). Summers et al. (2003) led a longitudinal study with patients diagnosed with CD (n = 4) and UC (n = 3). They administered participants with a single dose of 2500 live Trichuris suis eggs and followed them for 12 weeks. Remission was observed in three of the CD patients, a significant positive clinical response in the fourth CD patient, and all UC participants displayed a decrease in the Clinical Colitis Activity Index score. Multiple doses needed to be administered in order to maintain progress. The number of similar encouraging findings is growing (Arai & Lopes, 2022; Maruszewska-Cheruiyot, Donskow-Łysoniewska, & Doligalska, 2018; Weinstock & Elliott, 2013).
Dietary Treatments
Multiple dietary recommendations to treat IBD have been gaining in popularity. Tomasello et al. (2015) outlined benefits of dietary interventions: removing the side effects of drugs, reducing nutrient deficiencies and their physiological impacts, and promoting anti-inflammatory reactions.
Dairy is often considered inflammatory, but recent results seem to suggest it might be anti-inflammatory in people without intolerance (Labonté, Couture, Richard, Desroches, & Lamarche, 2013; Lucendo & Rezende, 2009; Nieman, Anderson, & Cifelli, 2020). The difference between raw milk and pasteurised milk, and between unfermented and fermented dairy products should be noted. Indeed, there are more significant amounts of psychotropic and mesophilic bacterial populations in raw milk than pasteurised, and they have been suggested to have a positive impact on allergies (van Neerven, Knol, Heck & Savelkoul, 2012), immunity, and health (Baars, Berge, Garssen, & Verster, 2019). The bacteria found in pasteurised milk are nonculturable (Quigley et al., 2013a,b). Yet, cultured (fermented) dairy products have been proven to be salutogenic in autoimmune diseases (Baars et al., 2019; Lerner & Matthias, 2018).
As Tomasello et al. (2015) mention, “data about the consumption of fibre are controversial” (p.84). Research seems to suggest two opposite opinions about fibre. On the one hand, fibre can act as a prebiotic and strengthen beneficial bacterial communities, and on the other hand, fibre is irritating, leading to diarrhoea and overall aggravation of inflammation in IBD. Deficiencies in microbes that can metabolise fibre to short chain fatty acids may lead to irritation by the unmetabolized fibre (Lucendo & Rezende, 2009; Tomasello et al., 2015).
Sugar and carbs are commonly accepted to be inflammatory (Aeberli et al., 2011; Cheng et al., 2021; Ma et al., 2022). Several authors have found that low-carb, low-sugar diets are effective in treating IBD (Lucendo & Rezende, 2009). A ketogenic diet, which removes all forms of sugar and carbs and replaces them with high levels of fat, resulting in ketone-based rather than glucose-based energy production, has been reported to treat inflammation (Dupuis, Curatolo, Benoist & Auvin, 2015; Lu et al., 2018; Masino & Ruskin, 2013; Pinto, Bonucci, Maggi, Corsi & Businaro, 2018; Ruskin, Kawamura & Masino, 2009; Tóth, Dabóczi, Howard, J. Miller & Clemens, 2016).
In spite of the promising results concerning fat consumption and ketosis, the role of fat is still debated. Many authors blame animal fat and consider fat as a potential trigger of IBD (Sakamato et al., 2005; Tomasello et al., 2015) and gut dysbiosis (Mentella et al., 2020).
Yet, in more than one randomised placebo-controlled paper, fish oil has been proven to ameliorate the clinical state and improve gut mucosal histology, as well as protecting from future relapses (Calder, 2006; Lucendo & Reznde, 2009). These findings have made PUFAs (polyunsaturated fats) popular. However, an overabundant consumption of omega 6 fatty acids can be inflammatory. Western diets include more omega 6 than omega 3 fatty acids, with a ratio reaching 17/1. Yet, the ratio was thought to be much lower during human evolution (Simopoulos, 2002). Lowering the ratio through increased consumption of omega 3 PUFA has been suggested to prevent mortality in cardiovascular disease and cancer, have beneficial effects on asthma, and revert inflammatory diseases (Simopoulos, 2006). The exact ratio depends on the illness and the patient, but data suggests that the consumption of vegetable oils should be reduced, while the consumption of fish, nuts, seeds, and vegetables should be increased (Ananthakrishnan et al., 2014; Russel et al., 1998; Tomasello et al., 2015). Omega 6 fatty acids have been suggested to play a role in the onset of IBD because of their role in increasing leukotriene B4 (Lucendo & Rezende, 2009). Yet, the most promoted and present fats in our western society are these omega 6 fats (sunflower oils, corn oils, soybean oils,…), together with hydrogenated/trans fats (fast foods, processed foods), which also play a crucial role in inflammation and IBD (Ananthakrishnan et al., 2014; Hou, Abraham, & El-Serag, 2011; Lucendo & Rezende, 2009; Persson, Ahlbom, & Hellers, 1992, as cited by Wang, Lin, Zhao & Li, 2017). Saturated fats are conventionally viewed as unhealthy, because of their supposed role in increasing cholesterol levels, atherosclerosis and heart disease (German & Dillard, 2004; Keys, 1953). This hypothesis has been debunked (German & Dillard, 2004; Ravnskov, 2002). Wang et al. (2017) found in their meta-analysis that the association between fat intake and UC could not be demonstrated. Moreover, their analysis suggested that: (1) omega 6 fatty acids promote inflammation, (2) omega 3 fatty acids are protective, (3) the relative risk of fat consumption is lower for saturated fat intake compared to monounsaturated and polyunsaturated fats. Similarly, Ananthakrishnan et al., (2014), in their longitudinal, large-scale study, found that “total fat, saturated or unsaturated fat, or individual PUFAs did not influence risk of CD”. Finally, Basson et al. (2021) suggest that some saturated fats could exert anti-inflammatory effects similar to those of omega 3 fatty acids. Thus, apart from the unhealthy or imbalanced omega 6 fatty acids and trans fatty acids, fat does not seem to be a culprit in IBD, especially when taking into consideration the promising findings concerning ketogenic diets mentioned in the previous paragraph.
IBD patients have repeatedly taken part in trials involving supplementation of nutrients, such as folate, Vitamin C, Vitamin B12, selenium, zinc and iron (Carrier et al., 2003; Lucendo & Rezendo, 2009; Sakamato et al., 2005), with interesting results. However, oral supplementation, especially of iron, might be of limited efficacy (Lucendo & Rezende, 2009). Nutrients found in food, in their natural form, are better absorbed than synthetic supplements (Cencic & Chingwaru, 2010; Lichtenstein & Russell, 2005). The importance of a nourishing diet cannot be overestimated.
Many diets have been suggested for IBD treatment. The FODMAP diet eliminates all fermentable oligosaccharides, disaccharides, monosaccharides, and polyols, preventing them from feeding pathogenic bacteria (Tomasello et al., 2015). Other elimination diets such as gluten-free diets, anti-inflammatory diets, or specific carbohydrate diets (SCD) have been assessed with encouraging results (Mentella et al., 2020).
The Current Study
In this paper, we suggest the GAPS Nutritional Protocol as a dietary intervention for IBD. GAPS stands for Gut And Psychology/Physiology Syndrome. This protocol is based on the SCD diet developed by Sidney Haas, which has shown promising results in the last century, especially for inflammatory conditions of the gut (Cohen et al., 2014; Gottschall, 2020; Obih et al., 2016; Suskind et al., 2020). The SCD diet excludes all types of complex carbohydrates (grains, starchy vegetables) and all refined sugar and double sugars (disaccharides).
The SCD diet has been adapted by Natasha Campbell-McBride to create the GAPS Nutritional Protocol (Campbell-McBride, 2008). The Full GAPS Diet eliminates all processed foods (including processed sugar and vegetable oils), grains, starchy vegetables and legumes, and focusses on nutrient-dense foods. The GAPS Introduction Diet is designed for deeper healing: it is a stage-by-stage introduction of nourishing foods, starting from the easiest-to-digest to more difficult-to-digest foods, slowly working up to The Full GAPS Diet. As it is crucial to replace the pathogens in our gut with beneficial microbes, probiotics are central to the GAPS Nutritional Protocol, and can be found both in fermented foods and commercial preparations. The GAPS Diet differs from the SCD diet due to the integration of probiotic foods, nutrient-dense foods such as organ meats, meat stock, healthy animal fats, and special ways of cooking foods based on traditional diets from around the world.
Compared to other popular diets such as FODMAP, gluten-free diets, ketogenic diets, anti-inflammatory diets and the Mediterranean diet, the GAPS Nutritional Protocol covers crucial aspects: (1) the necessity of getting most nutrients from homemade fresh food rather than from supplements, (2) the necessity of removing all processed foods, (3) an emphasis on animal fat consumption, fat-soluble vitamins, fermented foods, meat stock and other collagen-rich foods to heal the damaged gut wall, (4) the necessity of addressing toxicity in one’s environment and toxic overload in the body. Please see our previous paper (Delaunay-Vagliasindi, Seneff, & Campbell-McBride, 2021) and GAPS books (Campbell-McBride, 2020. 2010) for a thorough description of the GAPS Nutritional Protocol.
The use of the GAPS Nutritional Protocol has been growing steadily around the world in the last twenty years and GAPS has been implemented successfully by many practitioners, with promising results for various auto-immune, inflammatory and developmental disorders (Toygar & Bakirhan, 2023). In this paper, we aim to review the efficacy of the GAPS Nutritional Protocol in treating IBD.
Methodology
Participants and Recruitment
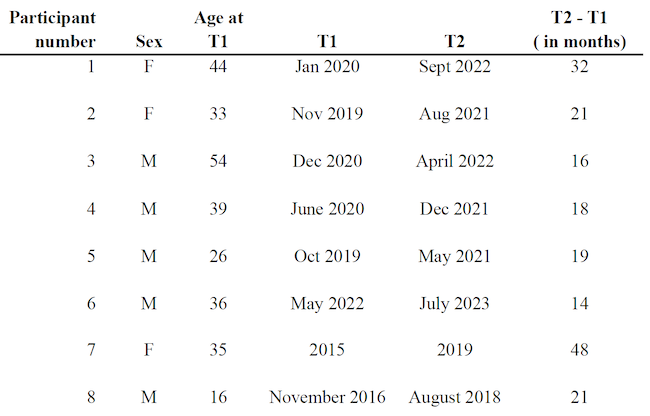
Participants were recruited through the database of experienced healthcare practitioners who use the GAPS Nutritional Protocol in their daily medical practice. The study was advertised on multiple platforms and through medical online conferences, calling for these certified practitioners to get in touch with their patients and participate in the study. An inclusion criterion was the official diagnosis of IBD prior to the implementation of the GAPS Diet. Consent forms guaranteeing anonymity were signed by all participants. No incentive was received by participants. The final sample consisted of eight participants (three females and five males). Mean age was 35.4. Six cases are discussed both quantitatively and qualitatively. Two participants were only interviewed and thus, discussed from a qualitative stance only (see Table 1 for complete demographics of the sample).
Table 1. Samples’ Information
.
Materials
Quantitative
The first six participants had taken lab tests when they first went to their Registered Dietician to implement the GAPS Nutritional Protocol. This was defined as Time 1 (T1).
They repeated lab tests after on average 20 months on the GAPS Nutritional Protocol (see Table 1). This was defined as our Time 2 (T2). In their blood counts, we focussed on two quantitative markers that are commonly accepted as significant in IBD : C-Reactive Protein (CRP) and Faecal Calprotectin (FC).
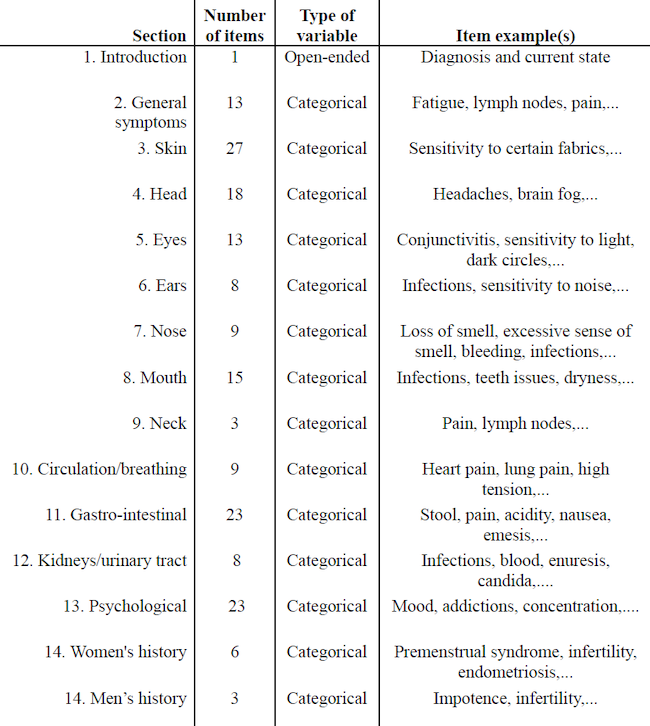
Four of these six participants also completed a thorough symptomatology questionnaire developed by Shantih Coro at both T1 and T2. The structured questionnaire consisted of 14 sections exploring patients’ absence or presence of general and specific health symptoms (see Table 2).
Qualitative
Two additional participants (participants 7 and 8) were interviewed retrospectively after having followed the GAPS Nutritional Protocol. T2 was defined as the time at which they reached out to us.
Table 2. Questionnaire’s Items
.
Categorical answers were trichotomous: people had to select whether the symptom occurs/occurred: (1) in the past (2) now (3) in the past and now.
Procedure
Quantitative
For both measures (CRP and FC), we compared means at T1 and T2 (see Table 3).
We also consistently categorised CRP levels and FC levels in terms of age-appropriate reference ranges for the markers (see Table 4). This allowed us to not only look at the increase or decrease of these blood count values but also assess their normal (or abnormal) levels.
Regarding the questionnaire, we looked at the average number of reported symptoms at T1 and T2 to see whether it decreased. We also looked at the average of gastro-intestinal symptoms specifically, i.e. section 11 of the questionnaire (see Table 2), since our sample consists of IBD patients who will be primarily affected in that area.
Qualitative
Individual answers to the questionnaire are discussed, to look at each participant’s individual evolution. The two additional interviews lasted one hour and were unstructured. Little to no prompting was given by the interviewer to ensure that the participant was not biassed in the elements they chose to report. The only instruction was to report their experience with the GAPS Nutritional Protocol and describe their current health status. Interviews were recorded.
Quantitative Data
CRP and FC Levels
Means were calculated only when data were available for both T1 and T2 (see Table 3). CRP mean level did not vary (M at T1 = 0.21; M at T2 = 0.22; n = 4). FC levels decreased (M at T1 = 833.33 ; M at T2 = 215.35; n = 6). CRP levels were all normal to begin with, while FC levels were abnormal in five out of the six participants at T1. At T2, they were only abnormal in two participants.
Questionnaire
For the four participants who completed the structured questionnaire at T1 and T2, the average number of reported symptoms decreased both when looking at the 14 sections (see Table 2) and when looking at the gastro-intestinal section of the questionnaire (see Figure 1 and Figure 2).
Table 3. CRP and FC Levels at T1 And T2
.
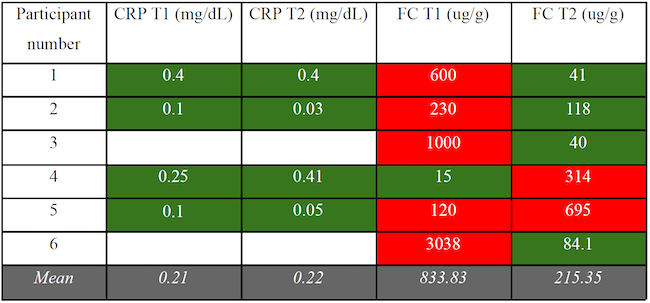
Colour chart: Red cells indicate a value significantly higher than normal for the relevant age range; Green cells indicate a value within normal range. Cells were left blank for missing data.
Figure 1. Mean Number of Reported Symptoms in Structured Questionnaire
.
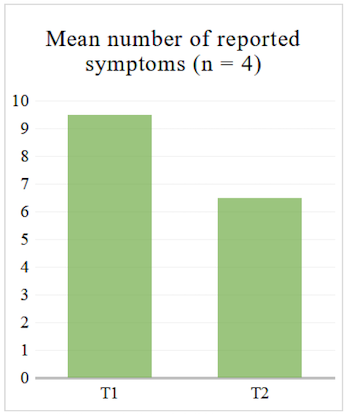
Figure 2. Mean Number of Reported Gastro-Intestinal Symptoms in Structured Questionnaire
.
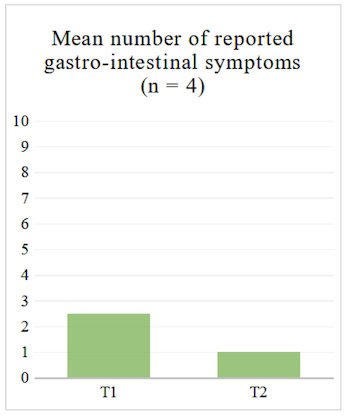
Qualitative Analysis
A Sample of Two Interviews
Participant 7
This participant is a 43 year old woman. She was nine years old when she experienced her first extreme abdominal pain and 20 years old when she was diagnosed with Crohn’s disease. Her mother was prone to anxiety. Her father had anaphylactic allergies to multiple foods.
She was breast-fed as a baby, but as soon as solids were introduced, she became very fussy with food. She remained a picky eater throughout her childhood and was suffering from constipation. She was very shy and had motion sickness. She experienced childhood trauma, which she did not disclose, with intergenerational family trauma among women in her family. She grew up in Poland and was exposed to radiation from Chernobyl’s explosion and toxic metals growing up in a coal mining town. There was black mould in her house. She believes these environmental factors have been crucial in her health history.
When she got diagnosed with Crohn’s disease at twenty, she had blood in her stool and alternated between profuse diarrhoea and constipation. Immunosuppressants helped for a while. For ten years, she was “in and out of hospitals” taking steroids and painkillers. Enemas were used for constipation. After some time, medications stopped helping.
The family lived in Poland, where a local Doctor called Janusz Pic was famous for his vegan protocol. Our participant decided to attend his vegan retreat. Her diet consisted of cooked vegetables, vegetable stocks, stews, soups and tofu. Everything was thoroughly cooked, nothing was consumed raw. No sugar or processed carbohydrates were allowed. Her digestive symptoms reduced, but she became clinically anorexic and severely underweight.
She became discouraged, thinking that “food makes no difference”. Thus, she went back to a more “standard” diet and drinking alcohol. Then she tried the SCD Diet, but there were many foods she could not tolerate. Her diet was very restricted. She says: “if I ate a raw vegetable, I would end up in the hospital. Banana, hospital. Nuts, hospital. Lots of blood, lots of cramping and extreme pain”. Stress also had a strong impact on her health.
She was taking steroid medications for years, but they stopped working and she was diagnosed with life-threatening Crohn’s disease. Her inflammation was constantly high and doctors did not know how to reduce it. “My gastroenterologist told me that I developed so much internal scarring around my ileum that it could burst at any time”. The doctors suggested Infliximab explaining that the drug will not reduce the size of her stricture but simply prevent further scarring. When she showed concern about the drug’s poor efficacy and heart-related side effects, her gastro-enterologist told her that it was “the only solution” for her and that there was medication to deal with heart disease if necessary. She was put under great pressure to take Infliximab.
This was a turning point that encouraged our participant to go back to food for healing. She started the GAPS Nutritional Protocol around her thirty-fifth birthday. She experienced a very powerful die-off reaction for three months with extreme brain fog and dysarthria (slurred speech). However, she was pain free and started sleeping better. “I knew I was onto something”.
She decided to try the No-Plant GAPS Diet, a version of the protocol that excludes all plant matter for people with severe gastro-intestinal inflammation. She consumed only animal products and increased animal fat consumption. She believes that the No-Plant GAPS Diet was the turning point for her. Since then, she has introduced new foods into her diet and consumes meat stock, meat, fish, animal fat, fermented dairy, as well as fermented vegetables. She also juices her vegetables or eats them well-cooked. After four years on the GAPS Protocol, she no longer suffers from any digestive issue.
She has been doing coffee enemas for years. At first, she had a taste of metal in her mouth after enemas, so she decided to explore chelation of toxic metals. She chelated very slowly with an experienced practitioner. Addressing her toxic metals load is what had the most profound impact on her panic attacks.
Concerning her mental state, she points out: “people reduce Crohn’s to digestive problems, but I have yet to meet someone who suffers from Crohn’s and does not have mental issues”. “You can do all the therapies you want, but if you do not heal your gut, your emotional state is not going to open up”. She tried many therapies, but it was not until her digestive system healed that she managed to heal emotionally. She reports significant reduction in her anxiety, panic attacks, arachnophobia, obsessive behaviours, mind fog, nightmares, depressive thoughts and mood swings. “I stopped blaming my parents and resenting them”. She considers herself to be 100% healed from a digestive stance and 90% healed from a psychological stance.
Her numerous physical health issues have also improved since implementing the GAPS Diet: yeast overgrowth, teeth grinding, gum sensitivity, and lack of sex drive have all dramatically reduced. Adrenal fatigue, anaemia, arthritis, premenstrual syndrome, clinical anorexia, sleep disorders, dandruff, eczema, vaginal thrush, and urinary tract infections have completely disappeared.
Participant 8
This participant is a 23 year old man. He experienced his first IBD symptoms three weeks after his sixteenth birthday in 2016. “I had blood in my stool. I was scared but also as a teenager I did not want to tell people”. He was diagnosed with ulcerative colitis and pancolitis. Doctors informed him that he would have to take medication for at least two years and that there was no cure for UC. Medication did not bring much improvement.
His symptoms were bad enough to stop him from going to school and he was entitled to receive home tuition. His mother started researching dietary treatments.
He followed the SCD Diet for four months with improvements. “November 2016 is the last time I ever ate chocolate”. This teenager felt so much better without eating processed sugars that he never re-introduced them. However, he still had IBD flare-ups. This is when they found the GAPS Nutritional Protocol.
He started with the GAPS Introduction Diet. During the first two weeks, he ate meat stock, raw eggs and sauerkraut juice. “It really relaxed my whole gastrointestinal tract”. Due to his condition he was underweight. He lost some weight initially, but his energy levels were “higher than ever”. He used to get hypoglycemia: he always had to carry something sweet (e.g. energy bars) in his bag because he was scared to faint. When he started the GAPS Nutritional Protocol, the hypoglycemia stopped. He was taking medication (Pentasa) which was causing side-effects (swollen feet, pain in his legs, difficulty swallowing…). The medication was stopped on the GAPS Introduction Diet. To this day he has never needed any other medication.
He followed the six stages of the GAPS Introduction Diet until he reached the Full GAPS Diet. By the time he turned 17, he had gained 15 kg, reaching average weight, and was back to being able to follow his high-intensity football training, dreaming of becoming a professional player. After one year and nine months on the GAPS Diet, all IBD symptoms had completely disappeared. In the last five years, he has never had a flare-up. He has been training intensively and considers himself “incredibly healthy”. He used to be an anxious teenager struggling with school and closed spaces, constantly worried about his weight, flare-ups and the future. Nowadays, he feels a lot more serene, and is planning to study physiotherapy in order to help others in an integrative way.
We had a short interview with his mother. We learned that, before 2016, our participant had always suffered from diarrhoea and was underweight. He was a colicky baby and did not sleep well. At nine months of age he started getting febrile convulsions and had to take paracetamol and ibuprofen every time he had a fever, which was quite frequent. He was breast-fed for almost two years, but took antibiotics and other medications for pneumonia and chest infections. His diet as a child was high in carbohydrates and sugars and low in fat. He would often feel weak and would eat sugar to keep his energy up. He also suffered from acid reflux during his childhood. His mother explained that he always craved meat, animal fats, eggs and butter, but she restricted these foods due to fear of heart disease. When he started the SCD Diet, and then the GAPS Nutritional Protocol, he was very happy to introduce these foods.
At the end of our interview, our participant said: “I just want to say I am so grateful to this protocol! There was no outlook for me and, as a sixteen year-old, that felt terrible”. The mother added: “ They [doctors] never even asked what he was eating before he became ill. And they are not interested in what he has been eating since his diagnosis. They know that he is not taking any medications, but they don’t want to learn anything about our dietary intervention which obviously led to his healing. One doctor said that he was really lucky that his colitis went away. I don’t think it had anything to do with luck. It was the work we were willing to invest that supported his body with the healing of his intestines and his immune system”.
Results of the GAPS Protocol at a Glance
In addition to the four participants who showed reduced inflammation in their FC levels, all participants experienced a drastic improvement in QOL.
Participant 5 went from reporting 13 symptoms at his T1 to reporting none at his T2. His initial symptoms included dry skin, recurrent nose infections and colds, swollen lymph nodes, stomach pain, constipation, blood in stool,… All these issues disappeared after a year and a half on the GAPS Nutritional Protocol. In the open-ended section of the questionnaire, he said he now feels “amazing”.
Participant 2, in the open-ended section of the questionnaire, says: “Relative to the symptomatology of my UC, I feel much better than when I started the diet with Dr. Coro. I manage the symptoms without the use of immunosuppressants unlike at the beginning, thanks to the GAPS Diet and lifestyle”. Her sensitivity to skin creams and fabrics had disappeared at T2, and her PMS had completely gone. She also no longer has blood in her stool.
Participants 3 and 4 wrote in their open-ended section that they feel “quite well”. In the reported symptoms, we observed that participant 3 got rid of skin issues and refound his sex drive, which he reported as nonexistent at T1. Participant 4 got rid of multiple gastrointestinal symptoms (cramps, gas, abnormal stool) as well as severe joint pain.
Participant 6 went back to a normal weight for his height and age after years of being underweight. He stopped having blood in his stool. Healing his IBD has also allowed him to dedicate some time to more emotional healing.
Participant 7 exited a life-threatening situation where multiple doctors had to regularly discuss her case due to her constant hospitalisations as soon as she consumed foods she could not tolerate (mainly nuts and vegetables). She is no longer clinically anorexic, has no gastro-intestinal issues, and discontinued the numerous immunosuppressants and biologic medicines that she was constantly prescribed. Many of her psychological and cognitive symptoms such as misophonia, phobias, and OCD, resolved after she implemented the GAPS Nutritional Protocol. She used to be so obsessed with and afraid of spiders that she constantly woke up due to nightmares. She struggled to travel to new places, as she had to check the bed for spiders multiple times and was afraid that there may be more varieties of spiders in the locations she visited. Now she can share more quality time with her partner.
Participant 8 is now ready to go back to school after having to drop out due to his gastrointestinal symptoms and his anxiety. He is excited about the future while he used to be highly anxious. His IBD does not prevent him from practising his favourite sport anymore, and he is considering moving abroad to become a physiotherapist.
Finally, all participants have discontinued their medications.
Discussion
Main Findings
This study aimed to investigate whether the GAPS Nutritional Protocol is a promising dietary intervention for IBD. All participants were diagnosed with either UC or CD. Participants 6 and 8 were also diagnosed with pancolitis.
IBD-related symptoms improved in all participants, and disappeared completely in five. This was visible in both our quantitative and qualitative data.
CRP levels were normal at T1 and remained normal at T2. On the other hand, FC levels were particularly sensitive to the implementation of the GAPS Nutritional Protocol. FC is among the most sensitive markers of IBD and distinguishes it from other disorders (Mumolo et al., 2018). This is consistent with our sample, given that CRP levels were not problematic to begin with, as opposed to FC levels. The mean FC levels decreased drastically after implementing the GAPS Nutritional Protocol, suggesting a reduction in inflammation. One of the participant’s FC levels went up, but his T2 was collected shortly after he had contracted COVID-19, which could have biassed the result concerning this marker (Udeh et al., 2021). When he more recently took a home-test (Patris Health, 2023) it turned out to be negative, suggesting that his FC levels went back to the normal range after the implementation of the GAPS Nutritional Protocol. Aside from this participant, there was only one other participant whose FC levels did not decrease (participant 4). This participant, even though he reported getting rid of multiple IBD symptoms (diarrhoea, cramps, gas, joint pain), is the only participant in our sample who does not consider himself healed yet or in remission. However, he finds the improvements encouraging enough to continue with the diet and hopes to see more improvements in the future.
Answers to the questionnaire suggested both overall (average reduction of reported symptoms in the sample) and individual (case-specific analysis of the open-ended questions) improvement. Implementing the GAPS Nutritional Protocol also had a positive impact on a plethora of comorbidities (see Figure 3).
The two unstructured interviews revealed complete healing of IBD and overall improvement of QOL. This improvement in QOL is a consistent result in all of our data.
Analysis of the Results
IBD, together with all chronic inflammatory conditions, has a high autoimmune component. In the mainstream, autoimmunity is explained by a theory of a molecular mimicry phenomenon, stating that the body attacks itself by mistake (Davidson & Diamond, 2001, p.340; Wang, Wang, & Gershwin, 2015, p.370). However, recent research is debunking the idea that the body is misguided. Shaheen, Quraishi, and Iqbal (2022) describe how gut dysbiosis leads to increased toxicity in the body produced by pathogenic microbes in the gut. Research in microbial transglutaminases demonstrates how toxins produced by pathogenic microbes in the gut attach themselves to proteins in the gut wall, contaminating them and changing their structure. The immune system responds to this contamination with inflammation and autoimmunity, leading to the clinical picture of IBD (Lerner & Matthias, 2020, 2019a, 2019b; Lerner et al., 2019). This contamination also occurs due to compromised gut lining (i.e. leaky gut syndrome). Xu et al. (2021) found hyposulfataemia and low mucin sulfation to play an important role in damaged intestinal epithelial tissues. These deficiencies can increase sensitivity to luminal toxins as well as increase inflammation (Dawson et al., 2009). Thus, the body does not attack its own tissues by mistake, but tries to deal with toxic contamination of its proteins in the gut wall. To assist the body in this process, it is essential to stop the contamination through restoring the normal microbial composition of the gut flora and healing the gut wall. Our results suggest that the GAPS Nutritional Protocol does this quite effectively. This can be explained by multiple factors.
First, the GAPS Nutritional Protocol removes ultraprocessed foods. Ultraprocessed foods have a negative impact on gut health and play a role in both inflammatory states and metabolic syndromes (Cuevas-Sierra, Milagro, Aranaz, Martínez, & Riezu-Boj, 2021; Zinöcker & Lindseth, 2018).
Figure 3. Improvement of Gastro-Intestinal Symptoms and Comorbidities
.
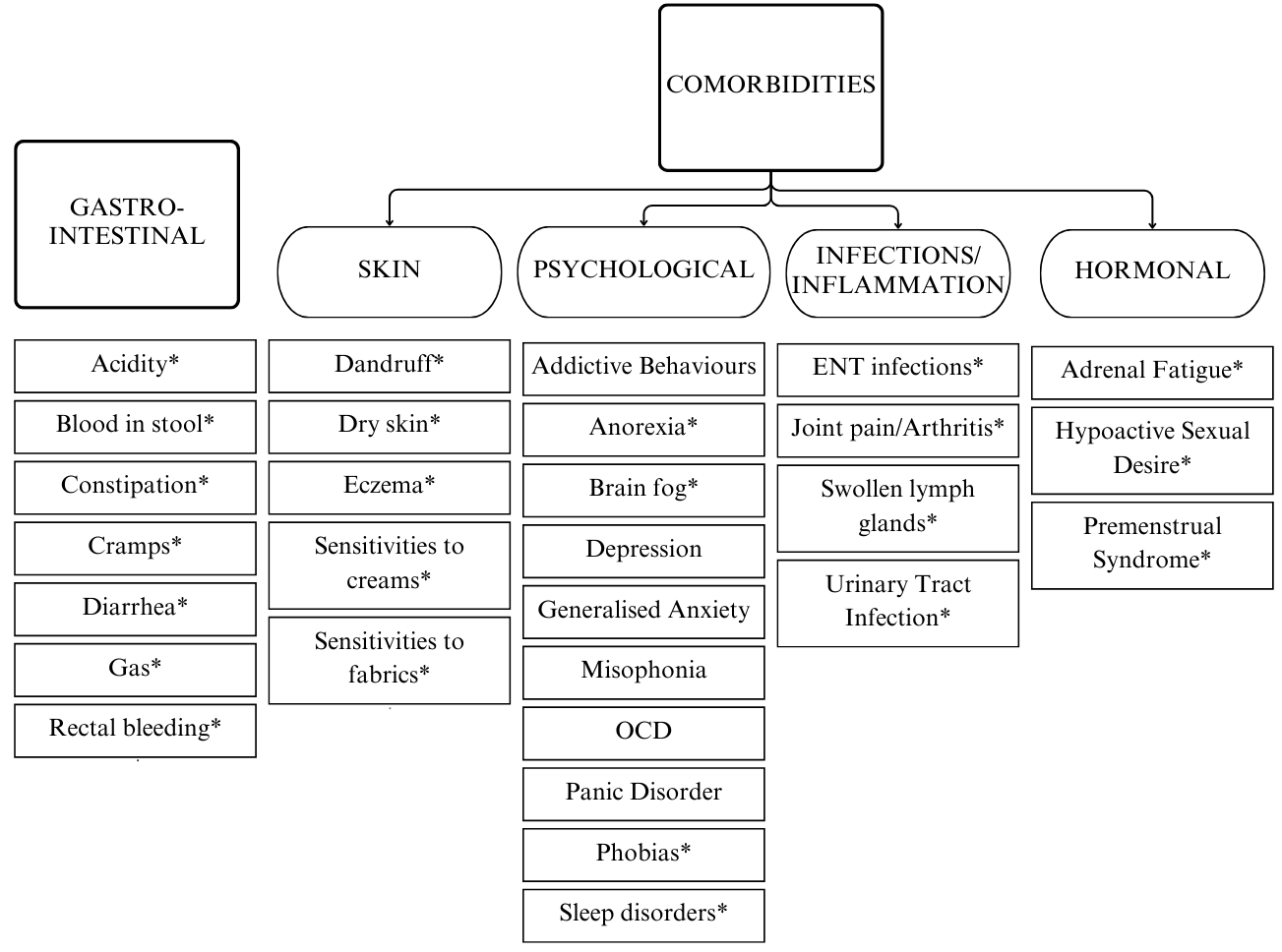
Overview of all symptoms that have improved or completely resolved during the implementation of the GAPS Nutritional Protocol. Those followed by an asterisk have completely resolved in more than one participant.
Second, it removes industrial sugars. Metabolic syndrome, insulin resistance and inflammatory responses are all intrinsically linked with high consumption of added sugars such as sucrose or high fructose corn syrup (Stanhope, 2015). This is highly relevant since research has found that IBD patients are more insulin-resistant than controls, which further explains their chronically inflamed state (Dogan et al., 2022; Korkmaz, Sahin, Ipekci, Temel, & Kebapcilar, 2014). Industrial sugars can also have a direct impact on gut microbiome composition. In their study, Kumar Jena & Prajapati (2016) found that rats who consumed a high sugar diet displayed increased coliforms and clostridium difficile strains. As discussed in this paper, these considerations do not only pertain to added processed sugar but also carbohydrates and starch, which is why these are removed from the diet when implementing the GAPS Nutritional Protocol. Older research (Samaranayake, 1985), research on ketogenic diets, and clinical experience (such as the one reported in this case study) suggests that removing processed carbohydrates is beneficial to reduce inflammation.
Third, it insists on how crucial it is to remove toxic chemicals used in industrial agriculture. Agricultural chemicals have been found to alter gut microbiota, which according to various authors, could be an important mediating factor between environment and health (Giambò, Teodoro, Costa, & Fenga, 2021; Shehata et al., 2013). On this matter, Swanson, Leu, & Abrahamson’s (2014) found a strong positive correlation between the rise in glyphosate usage and the rise in IBD over time. Authors have found that glyphosate disrupts the tight junctions of epithelial cells, causing a leaky gut (Gildea, Roberts & Bush, 2017). Gut permeability, as discussed above, plays a role in the proliferation of inflammation and IBD pathogenesis (Camilleri, 2019; Michielan & D’Incà, 2015). The GAPS Nutritional Protocol insists on the importance of meat stock and connective tissues, rich in collagen, which have the ability to heal and seal this intestinal barrier (Xing et al., 2022).
Fourth, by including a large amount of fermented foods, both from vegetal sources (sauerkraut, beet kvass,…) and animal sources (home-made yoghourt, kefir, sour cream), the GAPS Nutritional Protocol directly addresses gut dysbiosis and further assists in healing from auto-immune conditions. In our paper, we focused on dairy fermented for more than 24 hours, which has been found to be beneficial in chronic health conditions by multiple authors (Baars et al., 2019; Lerner & Matthias, 2018). Fermented cabbage has high concentration of lactic acid bacteria per gram, rendering it a probiotic superfood (Orgeron, Corbin, & Scott, 2016). Fermentation of plant foods enhances their digestibility, increases their richness in bioavailable nutrients, and makes them a valuable source of probiotics and enzymes (Campbell-McBride, 2020). This is illustrated in our sample; many participants struggled with vegetables, which often led to flare-ups. However, fermented vegetables were much better tolerated and provided important nutrition. Various studies have suggested that ingesting probiotic micro-organisms through natural home-made fermentation induces changes in gut composition which could explain these beneficial effects (Dimidi, Cox, Rossi, & Whelan, 2019; Jeong et al., 2017). In addition to the beneficial bacterial strains which might improve gut dysbiosis, fermented products also contain lactate. Spencer et al. (2022) found that this by-product of lacto-fermentation can increase microbiota-dependent Regulatory T-cells, which regulate inappropriate immune responses.
Fifth, as discussed, omega-6 highly processed vegetable oils and trans-fatty acids are pro-inflammatory. This is why they are avoided in the GAPS Nutritional Protocol and replaced primarily with omega-3 fatty acids and animal fats. In the GAPS Nutritional Protocol patients are encouraged to consume large amounts of these fats daily. Our cells are made of fat and cholesterol to a large degree, especially epithelial cells (Nelson et al., 2008). These fats are crucial to cell regeneration and healing of the leaky gut (Campbell-McBride, 2020).
Finally, fibre is avoided. We discussed how data concerning fibre is controversial in IBD; many authors suggest it has irritating properties (Lucendo & Rezende, 2009;Tomasello et al., 2015). Our sample seems to suggest that removal of fibre is beneficial and allows the digestive tract to heal and seal.
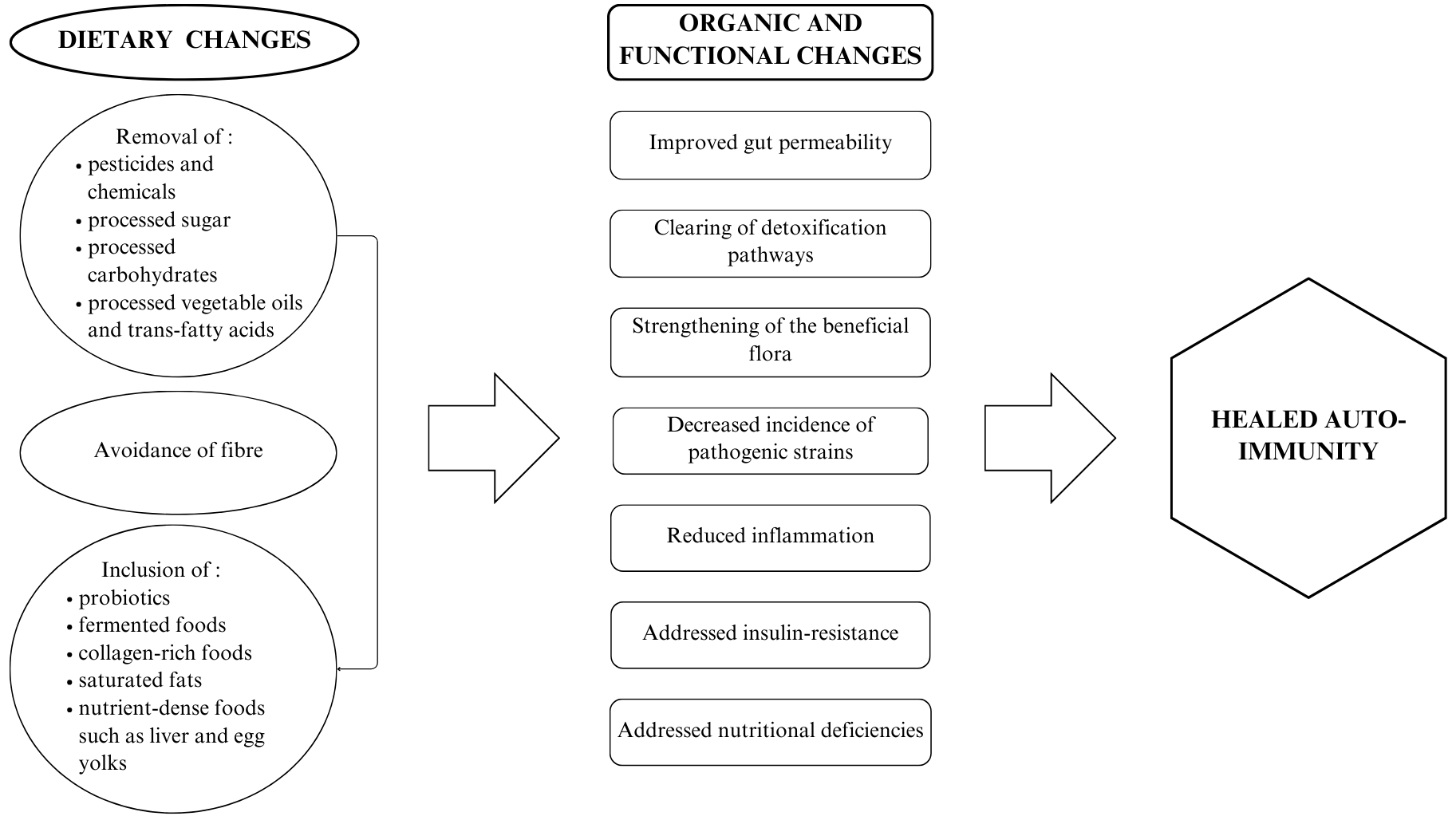
In sum, the GAPS Nutritional Protocol appears to address the root cause of IBD – gut dysbiosis and endogenous and exogenous toxicity – while providing correct and complete nutrition in order for the body to be able to heal (see Figure 4).
Figure 4. Holistic Impact of The GAPS Nutritional Protocol
.
Contributions
The Need for Treatments that Address the Cause of the Disease
There is a need to improve IBD management by shifting from medication-based symptomatic treatments to truly tackling the inflammatory mechanisms behind IBD (Lucendo & Rezende, 2009). Most importantly, multiple authors insist on including nutrition in IBD management (Tomasello et al., 2015).
We believe our paper brings additional support to this urgency, as the GAPS Nutritional Protocol does precisely that; it reduces inflammation without the need for medication. Medication reduces symptoms but does not treat IBD. In fact, commonly prescribed medications for IBD show a tendency to increase the inflammatory state in the body and further damage the gut lining (Lozupone et al., 2012).
GAPS Nutritional Protocol Versus Other Diets
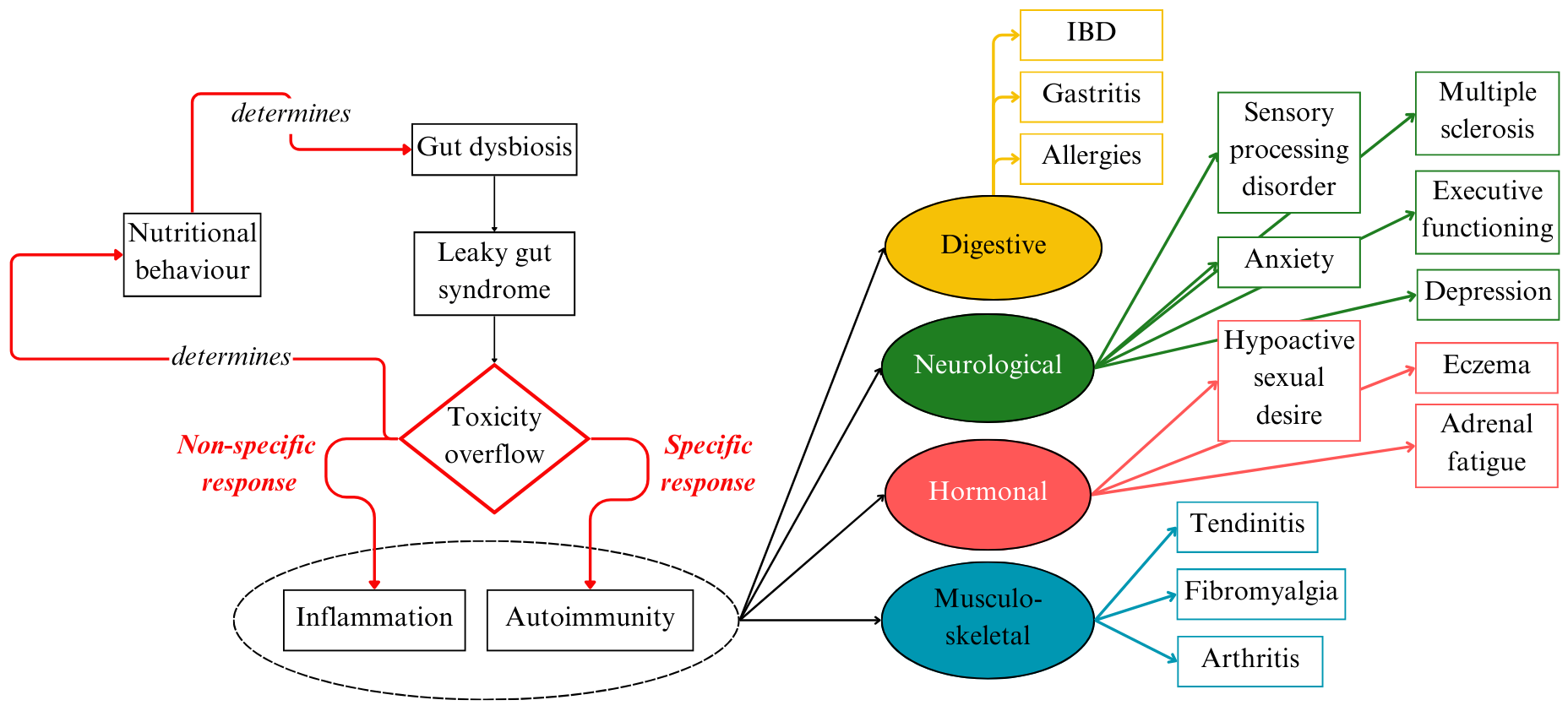
The GAPS Nutritional Protocol supports a growing body of scientific evidence that gut dysbiosis is the major source of inflammation and toxicity in the body. Addressing microbial imbalance in the gut should be the first consideration in chronic inflammation and autoimmune disease (see Figure 5).
A number of dietary treatments are proposed to address gastro-intestinal health (gluten-free, casein-free, SCD, FODMAP,…). Compared to other diets, the GAPS Nutritional Protocol covers crucial aspects: (1) the necessity of getting most nutrients from homemade fresh food rather than from supplements; (2) the necessity of removing all processed foods; (3) an emphasis on animal fat consumption, fat-soluble vitamins, fermented foods, meat stock and other collagen-rich foods to heal the damaged gut wall; (4) the necessity of addressing toxicity in one’s environment and toxic overload in the body (Campbell-McBride, 2020).
The GAPS Nutritional Protocol can be implemented with very positive results without supplementation. This is important, as many people cannot afford supplements. Supplements alone without a dietary change are not sufficient (Lichtenstein & Russell, 2005). To repair damaged and leaky gut lining, the body requires special nutrients provided in abundance by the GAPS Diet (see Figure 5) (Campbell-McBride, 2020).
In our sample, before finding the GAPS Nutritional Protocol, most participants had already tried other dietary interventions without success. Participant 7 was vegan for several months. She saw some initial improvements, but soon became severely underweight and fatigued. She also tried the SCD diet but many plant foods triggered flare-ups. Participant 6 was also vegan and underweight. He has now reached ideal BMI. Participant 8 tried the SCD diet; he felt better overall, but his energy level dropped and flare-ups continued. To quote him: “the GAPS Nutritional Protocol worked better than the SCD Diet for me because my body reactions were clear. I could clearly understand which foods I was reacting to”.
Figure 5. Underlying Mechanisms of IBD and Comorbidities
.
Limitations and Future Research
Future research on the GAPS Nutritional Protocol needs to include larger samples and prospective methodologies. However, even though statistically weak and more prone to bias, retrospective case studies are valuable in providing first evidence towards a hypothesis (Talary & Goyal, 2020). In addition, Lazard and McAvoy (2020) suggest that, rather than focusing on complete objectivity, which is unattainable, researchers should aim to be reflexive. They define reflexivity as “a form of critical thinking which aims to articulate the contexts that shape the processes of doing research” (Lazard & McAvoy, 2020, p.160).
Approaching our study as we did can only lead to positive implications, as research and clinical experience suggest that the dietary recommendations made by the GAPS Nutritional Protocol help heal, detoxify and sustain the human body. We urge practitioners to consider the importance of gut health in any chronic disease. It is vital to realise that correct nutrition is the most successful and most affordable way to address gut dysbiosis, chronic inflammation and autoimmune disease. We believe in the importance of integrative medicine, combining both natural medicine and mainstream Western practices. Recent studies suggest that an increasing number of countries are developing policies to encourage the use of integrative medicine in clinical settings (Toygar & Bakirhan, 2023). We hope this trend continues, and we encourage researchers to keep exploring the relationship between diet, gut dysbiosis, and disease.
Declarations
Ethics Approval and Consent to Participate
Not applicable. The study is retrospective. The patients engaged in the GAPS Nutritional Protocol willingly, i.e. they were not prompted by researchers for the purpose of the article.
Consent for Publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patients. Copies of consent forms are available for review by the Editor of this journal.
Availability of Data and Materials
The datasets and materials used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Dr Natasha Campbell-McBride is the creator of the GAPS concept and the GAPS Nutritional Protocol.
Funding
No financial support has been received for the work reported.
Authors’ Contributions
Shantih Coro and Sofie Declercq collected the data. Sophie Delaunay-Vagliasindi collected additional qualitative data, analysed all data, designed and wrote the manuscript. Natasha Campbell-McBride, Stephanie Seneff and Ophélie Planckaert supervised and edited. All authors read and approved the final manuscript.
Acknowledgements
This paper was written following the CARE guidelines.
Authors’ Information
Sophie Delaunay-Vagliasindi holds two Master’s degrees in Psychology (one MSc degree in Developmental Psychology from the University of Kent, UK, and another MSc degree in Clinical Psychology from the University of Louvain-la-neuve, Belgium). She is specialising in the impact of gut flora on development and conducting research for non-profit organisations, while working with patients in a clinical setting.
Stephanie Seneff is a Senior Research Scientist at Massachusetts Institute of Technology (MIT) in Cambridge, MA, USA. She holds a B.S. degree from MIT in biology, and a Ph.D. in electrical engineering and computer science also from MIT. Her recent research interests are on the role of nutritional deficiencies and toxic chemicals in disease, with a focus on the mineral sulphur and the herbicide glyphosate.
Shantih Coro holds a B.S in Nutrition and Dietetics from the University of North Florida, a M.S. in Exercise Science and Sports Nutrition from the University of Tampa and is currently pursuing a M.S. in Sports Nutrition from the Escuela Universitaria Real Madrid. Apart from working in the fitness industry and with high performance athletes, he is an experienced Certified GAPS Practitioner.
Ophélie Planckaert has a combined Bachelor’s Master’s (BS/MS) degree in agrifood science (ENITAC, Clermont-Ferrand, France) and also holds a Master’s degree in Biological Sciences (Université Laval, QC, Canada). She works as a researcher in non-profit organisations on the relationships between gut health and disease.
Sofie De Clercq holds a BSc in Nutrition and Dietetics at Hogeschool Gent, campus Vesalius and a post-BSc in Sports & Nutrition at Antwerpen Hogeschool. She is a Certified GAPS practitioner and has a private practice with a focus on gut health, digestive issues and fertility.
Natasha Campbell-McBride is a medical doctor with two postgraduate degrees: MMedSci (neurology), MMedSci (human nutrition). She is the creator of the GAPS concept and the GAPS Nutritional Protocol, described in detail in her two books Gut And Psychology Syndrome (2nd edition 2010) and Gut And Physiology Syndrome (2020).
References
Aeberli, I., Gerber, P. A., Hochuli, M., Kohler, S., Haile, S. R., Gouni-Berthold, I., . . .Berneis, K. (2011). Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. The American Journal of Clinical Nutrition, 94(2), 479-485. https://doi.org/10.3945/ajcn.111.013540
Aktories, K. (2011). Bacterial protein toxins that modify host regulatory GTPases. Nature Reviews Microbiology, 9(7), 487–498. https://doi.org/10.1038/nrmicro2592
Ananthakrishnan, A. N., Khalili, H., Konijeti, G. G., Higuchi, L. M., de Silva, P., Fuchs, C. S., … & Chan, A. T. (2014). Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut, 63(5), 776-784. https://doi.org/10.1136/gutjnl-2013-305304
Andoh, A., Bamba, T., & Sasaki, M. (1999). Physiological and anti‐inflammatory roles of dietary fiber and butyrate in intestinal functions. Journal of Parenteral and Enteral Nutrition, 23, S70-S73. https://doi.org/10.1177/014860719902300518
Arai, T., & Lopes, F. (2022). Potential of human helminth therapy for resolution of inflammatory bowel disease: The future ahead. Experimental Parasitology, 232, 108189. https://doi.org/10.1016/j.exppara.2021.108189
Assumpção, I., Rodrigues, M., & Barbieri, D. (1999). Tratamento da retocolite ulcerativa inespecífica em criança com enemas contendo butirato: relato de caso. Arquivos De Gastroenterologia, 36(4), 238-243. https://doi.org/10.1590/s0004-28031999000400012
Baars,T., Berge, C., Garssen, J., & Verster, J. (2019). The impact of raw fermented milk products on perceived health and mood among Dutch adults. Nutrition & Food Science. https://doi.org/10.1108/NFS-12-2018-0347
Bach, J.-F. (2005). Infections and autoimmune diseases. Journal of Autoimmunity, 25, 74–80. https://doi.org/10.1016/j.jaut.2005.09.024
Basson, A. R., Chen, C., Sagl, F., Trotter, A., Bederman, I., Gomez-Nguyen, A., … & Rodriguez-Palacios, A. (2021). Regulation of intestinal inflammation by dietary fats Frontiers in immunology, 11, 604989. https://doi.org/10.3389/fimmu.2020.604989
Bedford, A., & Gong, J. (2018). Implications of butyrate and its derivatives for gut health and animal production. Animal Nutrition, 4(2), 151-159. https://doi.org/10.1016/j.aninu.2017.08.010
Belkaid, Y., & Hand, T. (2014). Role of the Microbiota in Immunity and Inflammation. Cell, 157(1), 121-141. https://doi.org/10.1016/j.cell.2014.03.011
Berrilli, F., Di Cave, D., Cavallero, S., & D’Amelio, S. (2012). Interactions between parasites and microbial communities in the human gut. Frontiers in Cellular and Infection Microbiology, 2. https://doi.org/10.3389/fcimb.2012.00141
Bewtra, M., Su, C., & Lewis, J. (2007). Trends in Hospitalization Rates for Inflammatory Bowel Disease in the United States. Clinical Gastroenterology And Hepatology, 5(5), 597-601.e1. https://doi.org/10.1016/j.cgh.2007.01.015
Bibiloni, R., Fedorak, R. N., Tannock, G. W., Madsen, K. L., Gionchetti, P., Campieri, M., … & Sartor, R. B. (2005). VSL# 3 probiotic-mixture induces remission in patients with active ulcerative colitis. Official journal of the American College of Gastroenterology| ACG, 100(7), 1539-1546. https://doi.org/10.1111/j.1572-0241.2005.41794.x
Burisch, J., & Munkholm, P. (2015). The epidemiology of inflammatory bowel disease. Scandinavian journal of gastroenterology, 50(8), 942-951. https://doi.org/10.3109/00365521.2015.1014407
Camilleri, M. (2019). Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut, 68(8), 1516–1526. https://doi.org/10.1136/gutjnl-2019-318427
Calder, P. C. (2006). n− 3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American journal of clinical nutrition, 83(6), 1505S-1519S. https://doi.org/10.1093/ajcn/83.6.1505S
Campbell-McBride, N. (2020). Gut and Physiology Syndrome: Natural Treatment for Allergies, Autoimmune Illness, Arthritis, Gut Problems, Fatigue, Hormonal Problems, Neurological Disease and More. Medinform Publishing.
Campbell-McBride, N. (2008).Gut and Psychology Syndrome:Natural Treatment for Autism, Dyspraxia, A.D.D., Dyslexia, A.D.H.D., Depression, Schizophrenia. Medinform Publishing.
Carrier, J., Medline, A., Sohn, K. J., Choi, M., Martin, R., Hwang, S. W., & Kim, Y. I. (2003). Effects of dietary folate on ulcerative colitis-associated colorectal carcinogenesis in the interleukin 2-and β2-Microglobulin-deficient mice. Cancer Epidemiology Biomarkers & Prevention, 12(11), 1262-1267. https://aacrjournals.org/cebp/article/12/11/1262/167673/Effects-of-Dietary-Folate-on- Ulcerative-Colitis
Cencic, A., & Chingwaru, W. (2010). The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients, 2(6), 611-625. https://doi.org/10.3390/nu2060611
Cheng, W., Li, S., Lee, T., Lee, T., Chung, C., Kao, Y., & Chen, Y. (2021). Sugar fructose triggers gut dysbiosis and metabolic inflammation with cardiac arrhythmogenesis. Biomedicines, 9(7), 728. https://doi.org/10.3390/biomedicines9070728
Christl, S. U., Eisner, H. D., Dusel, G., Kasper, H., & Scheppach, W. (1996). Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa. Digestive diseases and sciences, 41(12), 2477-2481. https://doi.org/10.1007/BF02100146
Cohen, S. A., Gold, B. D., Oliva, S., Lewis, J., Stallworth, A., Koch, B., … Mason, D. (2014). Clinical and mucosal improvement with specific carbohydrate diet in pediatric crohn disease. Journal of Pediatric Gastroenterology and Nutrition, 59(4), 516–521. https://doi.org/10.1097/mpg.0000000000000449
Cohen, R. (2002). The quality of life in patients with Crohn’s disease. Alimentary Pharmacology &Amp; Therapeutics, 16(9), 1603-1609. https://doi.org/10.1046/j.1365-2036.2002.01323.x
Cuevas-Sierra, A., Milagro, F. I., Aranaz, P., Martínez, J. A., & Riezu-Boj, J. I. (2021). Gut microbiota differences according to ultra-processed food consumption in a Spanish population. Nutrients, 13(8), 2710. https://doi.org/10.3390/nu13082710
Dahlhamer, J. M., Zammitti, E. P., Ward, B. W., Wheaton, A. G., & Croft, J. B. (2016) Prevalence of inflammatory bowel disease among adults aged≥ 18 years—United States, 2015. Morbidity and mortality weekly report, 65(42), 1166-1169. https://www.jstor.org/stable/24859112
Davidson, A., & Diamond, B. (2001). Autoimmune diseases. New England Journal of Medicine, 345(5), 340–350. https://doi.org/10.1056/nejm200108023450506
Dawson, P. A., Huxley, S., Gardiner, B., Tran, T., McAuley, J. L., Grimmond, S., … Markovich, D. (2009). Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic nas1 null mouse. Gut, 58(7), 910–919. https://doi.org/10.1136/gut.2007.147595
Delaunay-Vagliasindi, S., Seneff, S., & Campbell-McBride, N. (2021). GAPS Nutritional Protocol: How healing the gut removes the basis for all chronic diseases. Journal of Orthomolecular Medicine, 36(3)
Dheer, R., Santaolalla, R., Davies, J. M., Lang, J. K., Phillips, M. C., Pastorini, C., … & Abreu, M. T. (2016). Intestinal epithelial toll-like receptor 4 signaling affects epithelial function and colonic microbiota and promotes a risk for transmissible colitis. Infection and immunity, 84(3), 798-810. https://doi.org/10.1128/IAI.01374-15
Dimidi, E., Cox, S. R., Rossi, M., & Whelan, K. (2019). Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients, 11(8). https://doi.org/10.3390/nu11081806
Din, ATU., & Alam, F. (2020). Auto-brewery syndrome: a clinical dilemma. Cureus, 12(10). https://doi.org/10.7759/cureus.10983
Doğan, A. N., Kahraman, R., & Akar, T. (2022). Evaluation of insulin resistance and beta cell activity in patients with inflammatory bowel disease. European Review for Medical & Pharmacological Sciences, 26(11).
Dupuis, N., Curatolo, N., Benoist, J., & Auvin, S. (2015). Ketogenic diet exhibits anti-inflammatory properties. Epilepsia, 56(7), e95-e98. https://doi.org/10.1111/epi.13038
Elinav, E., Strowig, T., Kau, A. L., Henao-Mejia, J., Thaiss, C. A., Booth, C. J., … & Flavell, R. A. (2011). NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell, 145(5), 745-757. https://doi.org/10.1016/j.cell.2011.04.022
Fasano, A. (2011). Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physio- logical reviews. https://doi.org/10.1152/physrev.00003.2008
Fasano, A. (2012) Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clinical Gastroenter- ology and Hepatology, 10(10), 1096-1100. https://doi.org/10.1016/j.cgh.2012.08.012
Fiocchi, C. (1998). Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology, 115(1), 182-205. https://doi.org/10.1016/S0016-5085(98)70381-6 Frank, D. N., Robertson, C. E., Hamm, C. M., Kpadeh, Z., Zhang, T., Chen, H., … & Li, E. (2011). Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflammatory bowel diseases, 17(1), 179-184. https://doi.org/10.1002/ibd.21339
Frank, D. N., St. Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N., & Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the national academy of sciences, 104(34), 13780-13785. https://doi.org/10.1073%2Fpnas.0706625104
German, J. B., & Dillard, C. J. (2004). Saturated fats: What dietary intake? The American Journal of Clinical Nutrition, 80(3), 550-559. https://doi.org/10.1093/ajcn/80.3.550
Gevers, D., Kugathasan, S., Denson, L. A., Vázquez-Baeza, Y., Van Treuren, W., Ren, B., … & Xavier, R. J. (2014). The treatment-naive microbiome in new-onset Crohn’s disease. Cell host & microbe, 15(3), 382-392. https://doi.org/10.1016/j.chom.2014.02.005
Giambò, F., Teodoro, M., Costa, C., & Fenga, C. (2021). Toxicology and microbiota: How do pesticides influence Gut Microbiota? A Review. International Journal of Environmental Research and Public Health, 18(11), 5510. https://doi.org/10.3390/ijerph18115510
Gildea, J. J., Roberts, D. A., & Bush, Z. (2017). Protective effects of lignite extract supplement on intestinal barrier function in glyphosate-mediated tight junction injury. Journal of Clinical Nutrition and Dietetics, 3(1), 1. https://doi.org/10.4172/2472-1921.100035
Gottschall, E. (2020). Breaking the vicious cycle. Kirkton Press.
Grisham, M. B. (1994). Oxidants and free radicals in inflammatory bowel disease. Lancet (London, England), 344(8926), 859–861. https://doi.org/10.1016/s0140-6736(94)92831-2
Hawthorne, A. B., Daneshmend, T. K., Hawkey, C. J., Belluzzi, A., Everitt, S. J., Holmes, G.K., … & Willars, J. E. (1992). Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut, 33(7), 922-928. https://doi.org/10.1136/gut.33.7.922
Helmby, H. (2015). Human helminth therapy to treat inflammatory disorders-where do we stand?. BMC immunology, 16(1), 1-5. https://doi.org/10.1186/s12865-015-0074-3
Hou, J. K., Abraham, B., & El-Serag, H. (2011). Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Official journal of the American College of Gastroenterology| ACG, 106(4), 563-573. https://doi.org/10.1038/ajg.2011.44
Jeong, D., Kim, D.-H., Kang, I.-B., Kim, H., Song, K.-Y., Kim, H.-S., & Seo, K.-H. (2017). Modulation of gut microbiota and increase in fecal water content in mice induced by administration of Lactobacillus kefiranofaciens DN1. Food & Function, 8(2), 680–686. https://doi.org/10.1039/c6fo01559j
Jostins, L., Ripke, S., Weersma, R. K., Duerr, R. H., McGovern, D. P., Hui, K. Y., … & Cho, J. H. (2012). Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature, 491(7422), 119-124. https://doi.org/10.1038/nature11582
Kaji H, Asanuma Y, Ide H, Saito N, Hisamura M, Murao M & Takahashi K (1976) The auto-brewery syndrome–the repeated attacks of alcoholic intoxication due to the overgrowth of Candida (albicans) in the gastroin- testinal tract. Materia medica polona, 8(4), 429-435.
Keys, A. (1953). Atherosclerosis: a problem in newer public health. Atherosclerosis, 1, 19.
Korkmaz, H., Sahin, F., Ipekci, S. H., Temel, T., & Kebapcilar, L. (2014). Increased pulse wave velocity and relationship with inflammation, insulin, and insulin resistance in inflammatory bowel disease. European Journal of Gastroenterology & Hepatology, 26(7), 725–732. https://doi.org/10.1097/meg.0000000000000104
Kumar Jena, P., & Prajapati, B. (2016). Influence of gut microbiota on inflammation and pathogenesis of sugar rich diet induced diabetes. Immunome Research, 12(1).
Labonté, M., Couture, P., Richard, C., Desroches, S., & Lamarche, B. (2013). Impact of dairy products on biomarkers of inflammation: A systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. The American Journal of Clinical Nutrition, 97(4), 706-717. https://doi.org/10.3945/ajcn.112.052217
Lane, E. R., Zisman, T. L., & Suskind, D. L. (2017). The microbiota in inflammatory bowel disease: current and therapeutic insights. Journal of inflammation research, 10, 63. https://doi.org/10.2147/jir.s116088
Lazard, L., & McAvoy, J. (2017). Doing reflexivity in psychological research: What’s the point? what’s the practice? Qualitative Research in Psychology, 17(2), 159–177. https://doi.org/10.1080/14780887.2017.1400144
Lee, Y. K., & Mazmanian, S. K. (2010). Has the microbiota played a critical role in the evolution of the adaptive immune system?. science, 330(6012), 1768-1773. https://doi.org/10.1126/science.1195568
Lerner, A., Wusterhausen, P., Ramesh, A., Lopez, F., & Torsten, M. (2019). The gut feeling of the joints: Celiac disease and rheumatoid arthritis are related. International Journal of Celiac Disease, 7.
Lerner, A., & Matthias, T. (2019a). Microbial transglutaminase: A new potential player in celiac disease. Clinical Immunology, 199, 37-43. https://doi.org/10.1016/j.clim.2018.12.008
Lerner, A., & Matthias, T. (2019b). Microbial transglutaminase is beneficial to food industries but a caveat to public health. Med One. https://doi.org/10.20900/mo.20190001
Lerner, A., Ramesh, A., & Matthias, T. (2019). The revival of the battle between David and Goliath in the enteric viruses and microbiota struggle: Potential implication for celiac disease. Microorganisms, 7(6), 173. https://doi.org/10.3390/microorganisms7060173
Lerner, A., & Matthias, T. (2018) The Salutogenic Effects of Cow’s Milk and Dairy Products in Celiac Disease. Journal Of Clinical & Cellular Immunology, 09(02). https://doi.org/10.4172/2155-9899.1000549
Ley, R. E., Peterson, D. A., & Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell, 124(4), 837-848. https://doi.org/10.1016/j.cell.2006.02.017
Li, J., Butcher, J., Mack, D., & Stintzi, A. (2015). Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflammatory bowel diseases, 21(1), 139-153. https://doi.org/10.1097/mib.0000000000000215
Lichtenstein, A. H., & Russell, R. M. (2005). Essential nutrients: Food or supplements? JAMA, 294(3), 351. https://doi.org/10.1001/jama.294.3.351
Lloyd-Price, J., Arze, C., Ananthakrishnan, A. N., Schirmer, M., Avila-Pacheco, J., Poon, T. W., … & Huttenhower, C. (2019). Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature, 569(7758), 655-662. https://doi.org/10.1038/s41586-019-1237-9
Looijer–Van Langen, M. A., & Dieleman, L. A. (2009). Prebiotics in chronic intestinal inflammation. Inflammatory bowel diseases, 15(3), 454-462. https://doi.org/10.1002/ibd.20737
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., & Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature, 489(7415), 220-230. https://doi.org/10.1038/nature11550
Lu, Y., Yang, Y. Y., Zhou, M. W., Liu, N., Xing, H. Y., Liu, X. X., & Li, F. (2018). Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neuroscience letters, 683, 13-18. https://doi.org/10.1016/j.neulet.2018.06.016
Lucendo, A. J., & De Rezende, L. C. (2009). Importance of nutrition in inflammatory bowel disease. World journal of gastroenterology: WJG, 15(17), 2081. https://doi.org/10.3748%2Fwjg.15.2081
Ma, X., Nan, F., Liang, H., Shu, P., Fan, X., Song, X., . . . Zhang, D. (2022). Excessive intake of sugar: An accomplice of inflammation. Frontiers in Immunology, 13. https://doi.org/10.3389/fimmu.2022.988481
Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., … & Vermeire, S. (2014). A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut, 63(8), 1275-1283. https://doi.org/10.1136/gutjnl-2013-304833
Maruszewska-Cheruiyot, M., Donskow-Łysoniewska, K., & Doligalska, M. (2018). Helminth Therapy: Advances in the use of Parasitic Worms Against Inflammatory Bowel Diseases and its Challenges. Helminthologia, 55(1), 1–11. https://doi.org/10.1515/helm-2017-0048
Masino, S. A., & Ruskin, D. N. (2013). Ketogenic Diets and Pain. Journal of Child Neurology, 28(8), 993–1001. https://doi.org/10.1177/0883073813487595
McGovern, D. P., Jones, M. R., Taylor, K. D., Marciante, K., Yan, X., Dubinsky, M., … & Rotter, J. I. (2010). Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Human molecular genetics, 19(17), 3468-3476. https://doi.org/10.1093%2Fhmg%2Fddq248
Mentella, M., Scaldaferri, F., Pizzoferrato, M., Gasbarrini, A., & Miggiano, G. (2020). Nutrition, IBD and Gut Microbiota: A Review. Nutrients, 12(4), 944. https://doi.org/10.3390/nu12040944
Michielan, A., & D’Incà, R. (2015). Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of Leaky Gut. Mediators of Inflammation, 2015, 1–10. https://doi.org/10.1155/2015/628157
Moller, F. T., Andersen, V., Wohlfahrt, J., & Jess, T. (2015). Familial risk of inflammatory bowel disease: a population-based cohort study 1977–2011. Official journal of the American College of Gastroenterology| ACG, 110(4), 564-571. https://doi.org/10.1038/ajg.2015.50
Moreels, T. G., & Pelckmans, P. A. (2005). Gastrointestinal parasites: potential therapy for refractory inflammatory bowel diseases. Inflammatory bowel diseases, 11(2), 178-184.
Morgan, X. C., Tickle, T. L., Sokol, H., Gevers, D., Devaney, K. L., Ward, D. V., … & Huttenhower, C. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome biology, 13(9), 1-18. https://doi.org/10.1186/gb-2012-13-9-r79
Mumolo, M. G., Bertani, L., Ceccarelli, L., Laino, G., Fluri, G. D., Albano, E., . . . Costa, F. (2018). From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World Journal of Gastroenterology, 24(33), 3681-3694. https://doi.org/10.3748/wjg.v24.i33.3681
Narula, N., Kassam, Z., Yuan, Y., Colombel, J. F., Ponsioen, C., Reinisch, W., & Moayyedi, P. (2017). Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflammatory bowel diseases, 23(10), 1702-1709. https://doi.org/10.1097/mib.0000000000001228
Nelson, D. L., Lehninger, A. L., & Cox, M. M. (2008). Lehninger principles of biochemistry. Macmillan.
Ngo, P. A., Neurath, M. F., & López-Posadas, R. (2022). Impact of Epithelial Cell Shedding on Intestinal Homeostasis. International Journal of Molecular Sciences, 23(8), 4160. https://doi.org/10.3390%2Fijms23084160
Nielsen, L. N., Roager, H. M., Casas, M. E., Frandsen, H. L., Gosewinkel, U., Bester, K., … & Bahl, M. I. (2018). Glyphosate has limited short-term effects on commensal bacterial community composition in the gut environment due to sufficient aromatic amino acid levels. Environmental Pollution, 233, 364-376. https://doi.org/10.1016/j.envpol.2017.10.016
Nieman, K. M., Anderson, B. D., & Cifelli, C. J. (2020). The effects of dairy product and dairy protein intake on inflammation: A systematic review of the literature. Journal of the American College of Nutrition, 40(6), 571-582. https://doi.org/10.1080/07315724.2020.1800532
Nieto, N., Torres, M. I., Ríos, A., & Gil, A. (2002). Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. The Journal of nutrition, 132(1), 11-19. https://doi.org/10.1093/jn/132.1.11
Obih, C., Wahbeh, G., Lee, D., Braly, K., Giefer, M., Shaffer, M. L., … Suskind, D. L. (2016). Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition, 32(4), 418–425. https://doi.org/10.1016/j.nut.2015.08.025
Orgeron, RP., Corbin, A., & Scott, B. (2016). Sauerkraut: A probiotic superfood. Functional Foods in Health and Disease, 6(8), 536-543. https://doi.org/10.31989/ffhd.v6i8.262.
Ott, S. J., Musfeldt, M., Wenderoth, D. F., Hampe, J., Brant, O., Fölsch, U. R., … & Schreiber, S. (2004). Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut, 53(5), 685-693. https://doi.org/10.1136/gut.2003.025403
Papadakis, K. A., & Targan, S. R. (2000). The role of chemokines and chemokine receptors in mucosal inflammation. Inflammatory bowel diseases, 6(4), 303–313. https://doi.org/10.1002/ibd.3780060408
Patris Health. (2023a July 6). Calprotectin Home Test. Retrieved from: https://www.patris-health.com/product/calprotectin-home-test/
Pinto, A., Bonucci, A., Maggi, E., Corsi, M., & Businaro, R. (2018). Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants, 7(5), 63. https://doi.org/10.3390/antiox7050063
Pithadia, A., & Jain, S. (2011). Treatment of inflammatory bowel disease (IBD). Pharmacological Reports, 63(3), 629-642. https://doi.org/10.1016/s1734-1140(11)70575-8
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., … & Wang, J. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. nature, 464(7285), 59-65. https://doi.org/10.1038/nature08821
Quigley, L., McCarthy, R., O’Sullivan, O., Beresford, TP., Fitzgerald, GF., Ross, RP., & Cotter, PD. (2013)a.The microbial content of raw and pasteurized cow milk as determined by molecular approaches. Journal of dairy science, 96(8), 4928-4937. https://doi.org/10.3168/jds.2013-6688
Quigley, L., O’Sullivan, O., Stanton, C., Beresford, TP., Ross, RP., Fitzgerald, GF., & Cotter, PD. (2013)b. The complex microbiota of raw milk. FEMS microbiology reviews, 37(5), 664-698. https://doi.org/10.1111/1574-6976.12030
Rausch, P., Rehman, A., Künzel, S., Häsler, R., Ott, S. J., Schreiber, S., … & Baines, J. F. (2011). Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proceedings of the National Academy of Sciences, 108(47), 19030-19035. https://doi.org/10.1073/pnas.1106408108
Ravnskov, U. (2002) Is atherosclerosis caused by high cholesterol?. Qjm, 95(6), 397-403 https://doi.org/10.1093/qjmed/95.6.397
Ruskin, D., Kawamura, M., & Masino, S. (2009). Reduced Pain and Inflammation in Juvenile and Adult Rats Fed a Ketogenic Diet. Plos ONE, 4(12), e8349. https://doi.org/10.1371/journal.pone.0008349
Russel, M. G., Engels, L. G., Muris, J. W., Limonard, C. B., Volovics, A., Brummer, R. J. M., & Stockbrügger, R. W. (1998). ‘Modern life’in the epidemiology of inflammatory bowel disease: a case-control study with special emphasis on nutritional factors. European Journal of Gastroenterology and Hepatology, 10(3), 243-250. https://doi.org/10.1097/00042737-199803000-00010
Sakamato, N., Kono, S., Wakai, K., Fukuda, Y., Satomi, M., Shimoyama, T., … & Epidemiology Group of the Research Committee on Inflammatory Bowel Disease in Japan. (2005). Dietary risk factors for inflammatory bowel disease A Multicenter Case‐Control Study in Japan. Inflammatory bowel diseases, 11(2), 154-163. https://doi.org/10.1097/00054725-200502000-00009
Samaranayake, L. (1985). On the role of dietary carbohydrates in the pathogenesis of oral candidosis. FEMS Microbiology Letters, 27(1), 1–5. https://doi.org/10.1016/0378-1097(85)90232-0
Sapone, A., De Magistris, L., Pietzak, M., Clemente, M., Tripathi, A., Cucca, F., & Fasano, A. (2006). Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes, 55(5), 1443-1449. https://doi.org/10.2337/db05-1593
Schmidt, G., Papatheodorou, P., & Aktories, K. (2015). Novel receptors for bacterial protein toxins. Current Opinion in Microbiology, 23, 55–61. https://doi.org/10.1016/j.mib.2014.11.003
Shaheen, W. A., Quraishi, M. N., & Iqbal, T. H. (2022). Gut microbiome and autoimmune disorders. Clinical and Experimental Immunology, 209(2), 161–174. £ https://doi.org/10.1093/cei/uxac057
Shehata, A. A., Schrödl, W., Aldin, A., Hafez, H. M., & Krüger, M. (2013). The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Current microbiology, 66(4), 350-358. https://doi.org/10.1007/s00284-012-0277-2.
Simopoulos, A. P. (2002). The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & Pharmacotherapy, 56(8), 365–379. https://doi.org/10.1016/s0753-3322(02)00253-6
Simopoulos, Artemis P. (2004). Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Reviews International, 20(1), 77–90. https://doi.org/10.1081/fri-120028831
Spencer, S. P., Silva, E. G. L., Caffery, E. B., Carter, M. M., Culver, R. N., Wang, M., … Sonnenburg, J. L. (2022). Fermented foods restructure gut microbiota and promote immune regulation via microbial metabolites. bioRxiv. https://doi.org/10.1101/2022.05.11.490523
Stanhope, K. L. (2015). Sugar consumption, metabolic disease and obesity: The state of the controversy. Critical Reviews in Clinical Laboratory Sciences, 53(1), 52–67. https://doi.org/10.3109/10408363.2015.1084990
Sturgeon, C. & Fasano, A. (2016). Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue barriers, 4(4), e1251384. https://doi.org/10.1080/21688370.2016.1251384
Summers, R. W., Elliott, D. E., Qadir, K., Urban Jr, J. F., Thompson, R., & Weinstock, J. V. (2003). Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. The American journal of gastroenterology, 98(9), 2034-2041. https://doi.org/10.1136%2Fgut.2004.041749
Suskind, D., Lee, D., Kim, Y., Wahbeh, G., Singh, N., & Braly, K. et al. (2020). The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn’s Disease: A Randomized Diet Controlled Trial. Nutrients, 12(12), 3749. https://doi.org/10.3390/nu12123749
Swanson, N. L., Leu, A., Abrahamson, J., & Wallet, B. (2014). Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. Journal of Organic Systems, 9(2), 6-37. https://www.organic-systems.org/journal/92/JOS_Volume-9_Number-2_Nov_2014-S wanson-et-al.pdf
Talari, K., & Goyal, M. (2020a). Retrospective studies – utility and Caveats. Journal of the Royal College of Physicians of Edinburgh, 50(4), 398–402. https://doi.org/10.4997/jrcpe.2020.409
Tinsley, A., Ehrlich, O., Hwang, C., Issokson, K., Zapala, S., & Weaver, A. et al. (2016). Knowledge, Attitudes, and Beliefs Regarding the Role of Nutrition in IBD Among Patients and Providers. Inflammatory Bowel Diseases, 22(10), 2474-2481.
https://doi.org/10.1097/mib.0000000000000901
Tomasello, G., Abruzzo, A., Sinagra, E., Damiani, P., Damiani, F., Traina, G., … & Lo Monte, A. I. (2015). Nutrition in IBD patient’s: what are the prospects. Progress in Nutrition, 17(2), 79-86. https://www.mattioli1885journals.com/index.php/progressinnutrition/article/view/3667
Tóth, C., Dabóczi, A., Howard, M., J. Miller, N., & Clemens, Z. (2016). Crohn’s disease successfully treated with the paleolithic ketogenic diet. International Journal Of Case Reports And Images, 7(9), 570. https://doi.org/10.5348/ijcri2016102-cr-10690
Toygar, F., & Bakirhan, H. (2023). Can integrative, complementary alternative medicine, and integrative and functional nutrition practices have a place in nutrition management? DAHUDER Medical Journal, 3(4), 105–116. https://doi.org/10.56016/dahudermj.1353461
Udeh, R., Advani, S., de Guadiana Romualdo, L. G., & Dolja-Gore, X. (2021). Calprotectin, an emerging biomarker of interest in COVID-19: A systematic review and meta-analysis. Journal of Clinical Medicine, 10(4), 775. https://doi.org/10.3390/jcm10040775
United States Centers for Disease Control. National Health and Nutrition Examination Survey 2013-2014 Data Documentation, Codebook, and Frequencies Glyphosate (GLYP) – Urine (SSGLYP_H). Data File: SSGLYP_H.xpt. June, 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/SSGLYP_H.htm
Vagianos, K., Bector, S., McConnell, J., & Bernstein, C. N. (2007). Nutrition assessment of patients with inflammatory bowel disease. Journal of Parenteral and Enteral Nutrition, 31(4), 311-319. https://doi.org/10.117/0148607107031004311
van Neerven, RJ., Knol, EF., Heck, JM., & Savelkoul, HF. (2012). Which factors in raw cow’s milk contribute to protection against allergies?. Journal of Allergy and Clinical Immunology, 130(4), 853-858. https://doi.org/10.10/j.jaci.2012.06.050
Wang, F., Lin, X., Zhao, Q., & Li, J. (2017). Fat intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. Journal Of Gastroenterology And Hepatology, 32(1), 19-27. https://doi.org/10.1111/jgh.13416
Wang, L., Wang, F., & Gershwin, M. E. (2015). Human autoimmune diseases: A comprehensive update. Journal of Internal Medicine, 278(4), 369–395. https://doi.org/10.1111/joim.12395
Weinstock, J., & Elliott, D. (2013). Translatability of helminth therapy in inflammatory bowel diseases. International Journal For Parasitology, 43(3-4), 245-251. https://doi.org/10.1016/j.ijpara.2012.10.016
Wong, W. Y., Chan, B. D., Sham, T. T., Lee, M. M. L., Chan, C. O., Chau, C. T., … & Tai, W. C. S. (2022). Lactobacillus casei Strain Shirota Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Increasing Taurine-Conjugated Bile Acids and Inhibiting NF-κB Signaling via Stabilization of IκBα. Frontiers in Nutrition, 9. https://doi.org/10.3389/fnut.2022.816836
Wu, H. J., & Wu, E. (2012). The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes, 3(1), 4-14. https://doi.org/10.4161%2Fgmic.19320
Xing, L., Fu, L., Cao, S., Yin, Y., Wei, L., & Zhang, W. (2022). The Anti-Inflammatory Effect of Bovine Bone-Gelatin Derived Peptides in LPS-Induced RAW264.7 Macrophages Cells and Dextran Sulfate Sodium-Induced C57BL/6 Mice. Nutrients, 14(7), 1479. https://doi.org/10.3390/nu14071479
Xu, P., Xi, Y., Zhu, J., Zhang, M., Luka, Z., Stolz, D. B., … Xie, W. (2021). Intestinal sulfation is essential to protect against colitis and colonic carcinogenesis. Gastroenterology, 161(1). https://doi.org/10.1053/j.gastro.2021.03.048
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., … & Gordon, J. I. (2012). Human gut microbiome viewed across age and geography. Nature, 486(7402), 222-227. https://doi.org/10.1038/nature11053
Zinöcker, M. K., & Lindseth, I. A. (2018). The Western diet–microbiome-host interaction and its role in metabolic disease. Nutrients, 10(3), 365. https://doi.org/10.3390/nu10030365
Zioudrou, C., Streaty, RA., & Klee, WA. (1979). Opioid peptides derived from food proteins. The exorphins. Journal of Biological Chemistry, 254(7), 2446-2449. https://doi.org/10.1016/S0021-9258(17)30243-0