.
Food Triggers and Symptoms
Food Protein-Induced Enterocolitis Syndrome (FPIES) is a non-Immunoglobulin E (non-IgE) mediated response to foods generating severe gastrointestinal problems. Soy and cow’s milk are the most common trigger foods, but grains, nuts, meat, poultry, seafood, eggs, fruits and vegetables can also be culprits (Caubet et al., 2014; Delahaye, Chauveau, Kiefer, & Dumond, 2017; Halbrich, Ben-Shoshan, & Rex, 2014; Nowak-Węgrzyn, 2015; Ruffner et al., 2013; Sicherer, 2005). Patients experience vomiting with or without lethargy one to four hours after food ingestion, and diarrhea up to 24 hours later (Katz, Goldberg, Rajuan, Cohen, & Leshno, 2011; Lemoine et al., 2022; Nowak-Wegrzyn et al., 2017). These symptoms can lead to more severe complications such as hypotension (Coates, Weaver, Lloyd, Ceccacci, & Greenberg, 2011; Sicherer, 2005), methemoglobinemia, metabolic acidosis (Murray & Christie, 1993), dehydration or cardiac shock (Gryboski, 1967; Sicherer, 2005). Unlike anaphylaxis, patients with FPIES do not show respiratory or skin reactions to foods (Nowak-Wegrzyn et al., 2017), but can have higher risks of suffering from IgE mediated food allergies and other allergic conditions such as asthma, eczema, allergic rhinitis, latex allergy and urticaria (Caubet et al., 2014; Nowak-Wegrzyn et al., 2019).
Food Protein-Induced Allergic Proctocolitis, also known as allergic colitis or eosinophilic proctocolitis, is a non-IgE immune reaction, in response to intestinal inflammation (Mennini et al., 2020; Nowak-Wegrzyn et al., 2015). Most common symptoms include pronounced rectal bleeding and/or bloody stools, with possible mild diarrhea and absence of emesis (Aslan, Koca, & Akcam, 2017; Nowak-Wegrzyn et al., 2015). It affects young and apparently healthy infants, including exclusively breast-fed babies, and is mainly triggered by dairy products, although other foods such as eggs, meats, grains, nuts and fish can cause proctocolitis reactions (Aslan et al., 2017; Mennini et al., 2020; Yilmaz et al., 2017). Onset tends to occur earlier than for FPIES, first symptoms appearing usually within the first months of life (Mennini et al., 2020; Nowak-Wegrzyn et al., 2015; Yilmaz et al., 2017). Infants with multiple trigger foods are more likely to have used antibiotics and to have atopic dermatitis, wheezing, colic or IgE sensitivity than infants with a single trigger food (Buyuktiryaki et al., 2020).
Diagnosis
The diagnosis of FPIES relies heavily on the clinical picture: symptoms which appear following ingestion of specific foods and which disappear with the removal of these foods from the diet (Nowak-Wegrzyn et al., 2017). IgE testing on the culprit food can be helpful (Nowak-Wegrzyn et al., 2017), but cannot be the only criterion for the identification of FPIES, as IgE antibodies are detected in only 20% of patients (Caubet et al., 2014; Katz et al., 2011). For some FPIES patients, Shek et al. (2005) found detectable IgE antibodies to three individual caseins but not against whole cow’s milk, thus explaining the low level of IgE antibodies against real foods. As for FPIES, diagnosis of FPIAP is empirical, in the absence of a specific test; it is based on medical assessment, testing symptoms in an elimination diet (Mennini et al., 2020).
Management and Treatment
According to Nowak-Wegrzyn and collaborators (2020, p.25), “there are no strategies to accelerate development of tolerance in FPIES”. Recommendations have been made to limit the frequency of FPIES episodes, lessen the nutritional deficiencies and reduce the symptoms in the case of an accidental exposure. The international consensus guidelines for the diagnosis and management of FPIES recommend complete avoidance of trigger foods, varying of textures and flavours of tolerated foods, and consuming supplements to compensate for the lack of energy and vitamins (Nowak-Wegrzyn et al., 2017). During FPIES episodes, hydration is advised for low to moderate FPIES, while intravenous saline solution is recommended for severe cases (Nowak-Wegrzyn et al., 2017).
In exclusively breast-fed infants, treatment of FPIAP consists in eliminating presumed trigger foods from the mother’s diet; when restricting the mother’s diet is not sufficient to eliminate symptoms, or when the mother’s diet is too restricted, breast-feeding ceases and infants are exclusively fed with formula (Mennini et al., 2020). Bottle-fed children are given an extensively hydrolysed or amino acid-based formula (Aslan et al., 2017).
Tolerance Testing and Recovery
FPIES patients conduct oral food challenges to test their tolerance to foods, introducing one ingredient at a time in very small amounts, often under medical supervision (Caubet et al., 2014; Maloney & Nowak-Wegrzyn, 2007). Recovery rates vary with the food trigger, with a median age of tolerance ranging between 1 and 13.8 years old (Caubet et al., 2014; Katz et al., 2011).
In case of no food-specific IgE allergy additional to FPIAP, tolerance for culprit food in allergic proctocolitis is tested at home after 4 to 8 weeks of elimination (Nowak-Wegrzyn et al., 2015). FPIAP is commonly solved in one to three years (Cetinkaya, Ocak, Sahiner, Sekerel, & Soyer, 2021; Lake, 2000; Mennini et al., 2020) but can convert to FPIES (Taştan & Arslan, 2023).
Implementation of the GAPS Nutritional Protocol
In this paper we describe the story of seven children who recovered from FPIES and one child who recovered from FPIAP after having followed the GAPS Nutritional Protocol developed by Dr. Campbell McBride (Campbell-McBride, 2010, 2020). GAPS stands for Gut and Psychology/Physiology Syndrome. Its core principles rely on the need of a healthy gut microbiome for human metabolic health. The core of the GAPS Protocol is the GAPS Diet, based on ancestral diets from all over the world. In the GAPS Diet, starch, complex carbohydrates and all processed foods are removed (all grains, refined sugars, processed oils, starchy vegetables and legumes, etc). All food is prepared at home from natural ingredients: animal foods (meat, fish, eggs and fermented raw dairy), non-starch vegetables (many in a fermented form) and ripe fruits. Homemade meat stock and soup are a staple, while nuts and oily seeds are occasionally used for baking. This is the so-called “Full GAPS Diet”. Some patients with severe symptoms have to go through the GAPS Introduction Diet first, which is more restrictive and more difficult to follow but achieves faster healing. The GAPS Introduction Diet includes six stages, starting with the consumption of easy-to-digest foods only and slowly adding harder-to-digest foods into the diet, to finally reach the Full GAPS Diet. For patients with severe FPIES, a No-Plant variation of the GAPS Diet is used, during which patients consume only home-cooked animal-based products. All plant matter is avoided until the main symptoms subside. When the patient is stronger, plant foods are introduced slowly and very carefully, starting with fermented and well-cooked non-starchy vegetables. The different approaches of the GAPS Diet are described in detail in the book Gut And Physiology Syndrome by Dr. Campbell-McBride (2020). In the GAPS Nutritional Protocol, lifestyle changes are also implemented and a chemical-free environment is created for the patient. Natural food supplements can occasionally be used. GAPS guidelines include enjoying the sunshine without chemical protection during the warm season, swimming only in natural waters, and walking barefoot when possible.
The efficiency of the GAPS Nutritional Protocol in chronic diseases, both physical and mental, has been confirmed over the last 20 years through clinical experience and many testimonies. Published studies have demonstrated the effectiveness of the GAPS Nutritional Protocol in improving socialising, behaviour, tics, learning disabilities and PANDAS (Ābele, Meija, Folkmanis, & Tzivian, 2021; Delaunay-Vagliasindi, Seneff, & Campbell-McBride, 2021; Delaunay-Vagliasindi, Seneff, Coro, & Campbell-McBride, 2021; Delaunay-Vagliasindi, Seneff, Coro, Plotner, & Campbell-McBride, 2022). Toygar and Bakirhan (2023) revealed that the majority of Turkish nutritionists used and recommended the GAPS diet in functional medicine and nutrition.
In this paper, we suggest that the GAPS Nutritional Protocol can help people resolve FPIES, even in the most severe cases with multiple food triggers where conventional elimination diets have failed.
Methodology
Participants
Inclusion criteria were (1) Experience with FPIES or FPIAP, and (2) Past implementation of the GAPS Nutritional Protocol. Participants were recruited through the database of experienced healthcare practitioners who use the GAPS Nutritional Protocol in their practice.
The GAPS Nutritional Protocol is always adapted to each person: patients are taught to listen to their body and adjust the protocol accordingly. This explains why the stories reported below show different ways of implementing the GAPS Nutritional Protocol. However, the core of the protocol remains identical for all: elimination of processed foods from the diet, addition of fermented foods, animal fats, and gelatinous meats, together with lifestyle changes reducing toxic overload from the environment.
Four subjects were diagnosed with FPIES by medical doctors. The other four (cases 3, 4, 5 and 8) never received a medical diagnosis explicitly citing FPIES or FPIAP. However, cases 3, 4 and 8 had repetitive crises following the consumption of several foods with profuse emesis and severe lethargy. These symptoms are the diagnostic criteria for FPIES (Nowak-Wegrzyn et al., 2017), thus they were included in this paper as patients with FPIES. Case 5 presented the characteristic symptoms of FPIAP, i.e. very early onset with normal growth and absence of emesis combined with mild diarrhea and bloody stools following the consumption of the trigger food (Lake, 2000; Nowak-Wegrzyn et al., 2015); thus, it was included as a FPIAP case. See Table 1 for complete demographics.
Data Collection
Two methods were used to collect data. Some participants filled in a questionnaire by email including questions on health history, symptoms, diet, and lifestyle before and while on the GAPS Nutritional Protocol. Additional questions on the current diet of the child were asked. Other parents preferred sharing their story during a phone interview that was recorded with their agreement. Except for those on identity, questions were open-ended in order not to influence parents in their answers. Signed consent forms were obtained from all participants.
Results
Description of the Case Study
Eight families agreed to share their experience (seven with FPIES, one with FPIAP). All patients were born after at least eight months of pregnancy.
We report hereafter two cases, one for FPIES (case 4) and one for FPIAP (case 5). All other cases can be found in the appendix.
FPIES Case
This boy, born through natural delivery in 2008, was tested for allergies at 18 months because of severe reactions to foods. After ingesting the trigger food, he would cough up a lot of phlegm and then vomit until his stomach was empty. With age, his reactions evolved: before coughing phlegm and vomiting he would present hives first, always positioned around his major lymph glands. In a very severe crisis, the infant would become lethargic, his eyes would swell shut and his body would flare up with urticaria. His mother would immediately give him a magnesium salt bath to stop his reaction. His parents found a correlation between vaccinations and his progress in healing FPIES. Following each vaccine, the child would lose all safe food gains and start reacting to foods again.
At 18 months, allergy testing revealed many food allergies, including milk, eggs, all tree nuts, sesame seeds, meat, wheat, and vegetables. The family doctor, who was not familiar with the diagnosis of FPIES, diagnosed “eosinophilic esophagitis, anaphylaxis and food allergies”, prescribed hypoallergenic formula and a complete elimination diet. Doctors told the parents that their son should never eat any food, apart from a mixture of rice, pears, tinned sweet corn and sugar. The little boy was fed with a hypoallergenic formula for three and a half years. The infant was intolerant to all natural foods and was put on medication. He took up to seven medications at a time, including steroids. Then his mother discovered an online group of parents implementing the GAPS Nutritional Protocol with their children. She realised that her son had the same symptoms as FPIES children. She expressed relief in finally being able to understand her son’s health issues, and more importantly realize that they were curable. At that time, her son had 54 IgE-related or FPIES allergies.
Right after his fifth birthday, the boy’s parents introduced a few drops of lamb stock in their son’s formula. In three months, he became tolerant to meat stock and could drink half a bottle of mixed formula and meat stock every day. The boy quickly became tolerant to zucchini, butternut squash and carrot, and was able to start the GAPS Introduction Diet six months after his fifth birthday. He stopped taking his formula and morning sneezing and congestion immediately disappeared. His parents were astonished to realize that their son had been intolerant to his formula since the beginning.
Within four days on the GAPS Introduction Diet, the little boy could tolerate egg yolk, his most allergenic food. Within 11 days, he could tolerate almonds, his second most allergenic food. After six weeks on the GAPS Introduction Diet, the boy could tolerate many more foods, started gaining weight and became much healthier. “He had this beautiful colour to replace the pale white ghostly look”, says his mother. He was able to have Christmas lunch with his family for the first time, enjoying each mouthful of his GAPS food. When he reached the Full GAPS Diet a few months prior to his sixth birthday, he had overcome allergies to meat, organ meats, egg yolks, meat stock, soup, almond bread and many vegetables.
Progress was slower in the three following years as the child had to go through clearing parasites out of his body. At seven years of age, he had healed from FPIES and at nine years old all his anaphylactic allergies were gone. He also healed from eosinophilic esophagitis, sensory processing disorder (SPD) and asthma. He is now 14 years old, “glows with health” according to his mother, and is a healthy teenager about to attend university.
During the same period, the whole family followed the GAPS Nutritional Protocol, and his sister healed from food intolerances and attention-deficit/hyperactivity disorder. The family can now occasionally eat a pizza with no reaction and enjoys a natural lifestyle growing biodynamic food on their five-acre farm.
FPIAP Case
This boy of 16 months of age was born naturally. When he was three days old, his mother observed the same symptoms as for her older son: in addition to eczema, gas, discomfort and baby acne, the baby had watery dark green stools rich in mucus. When he was just ten days old, blood appeared in his stools.
His mother started removing foods from her own diet, as she was exclusively breastfeeding her son. She realized that chicken was giving him his worst symptoms (bloody stools), and that her son tolerated one single food through her breast milk – lamb meat. The infant would react to all other foods with colic, stools rich in mucus and eczema flares. He would get constipated alternating with very runny stools. The only time the infant had a dairy product (sheep yoghurt), he developed bloody stools.
When the child was six months old, his parents discovered the GAPS Nutritional Protocol and started implementing it. The only food that was given to the infant was lamb meat stock and soup, staying on stage one of the GAPS Introduction Diet for two months. Vegetables created allergic reactions. When the reactions lessened, fermented food was introduced into the boy’s diet, but this worsened his eczema and created abnormal stools. However, his parents knew they were feeding their son with nourishing food and pushed through. Always attentive to their son’s reactions, they kept increasing the amount of sauerkraut juice and milk kefir very slowly. After six months on stages one and two of the GAPS Introduction Diet, they tried adding eggs. Egg yolk was introduced and tolerated for one week while in the USA, but created a reaction when the family went back home to Canada. It was avoided for two months, before being added again into the child’s diet, starting with one drop. At that stage, the boy tolerated it well. After his first birthday, the toddler ate chicken and also tolerated it.
Despite better tolerance to many foods, the infant still had eczema. His parents implemented iodine painting, increased the child’s sunlight exposure as advised in the GAPS Protocol (gradually, not at warmest hours and without sunscreen) and increased the intake of milk kefir. After strong reactions for two to three weeks, eczema decreased by 90%.
Today, the child is on the Full GAPS Diet, with reduced amounts of fruits, nuts and raw vegetables. He eats a lot of meat, meat stock, milk kefir fermented for 24 to 30 hours, animal fat such as sour cream fermented for at least 48 hours, beet kvass, sauerkraut juice and cooked vegetables.
His food is always organic and he takes cod liver oil. His stools are now regular and normal.
The whole family has been following the GAPS Nutritional Protocol, and the parents and their three children have become much healthier. The older brother (three years old) used to cry a lot day and night, had hives, vomiting, eczema, blood in his stool and weight loss, going from 90% at birth to 20% on the weight curve. Following the GAPS Nutritional Protocol, he now sleeps better, puts on weight and is a far less picky eater than before. He also does not have blood in his stools anymore.
Their mother says that “GAPS is a commitment, but that [she is] glad to have discovered it.” She hopes that “more people will be aware of this protocol and will implement it to have healthier children”.
Analysis
Our cohort was made up of eight children (4 boys and 4 girls) mostly from North America and born between 2008 and 2022.
Based on the qualitative results reported above and in our appendix, we analysed four themes that emerged: (1) triggers, (2) symptoms, (3) duration of our intervention, (4) effect of breastfeeding and vaccinations.
Triggers
Five of seven children with FPIES (71.4%) showed reactions to multiple foods (Table 1). Egg was the most common FPIES food trigger (71.4%), followed by dairy products, chicken and sweet potatoes (57.1%) (Figure 1). In our cohort, 42.9% of children showed FPIES reactions to meats, avocado, mango, banana, other vegetables, tree nuts, sesame seeds, rice, wheat and/or oat (Table 1). One child (14.3%) reacted to absolutely all foods, and one child (14.3%) tolerated only two foods. The patient suffering from FPIAP reacted to all foods except lamb.
Symptoms
Our results reveal that symptoms vary a lot among FPIES patients, with a list of 27 different symptoms reported by parents (Table 2). However, some symptoms were more frequent than others. During FPIES episodes, the most common symptoms were emesis (85.7%), diarrhea, lethargy and paleness (71.4%) (Table 2). All members of the cohort had constant health problems, and more than half of the cohort suffered from eczema, weight loss (71.4%), anaphylactic reactions and/or sleep issues (57.1%).
Our participant with FPIAP experienced baby acne, bloody stools, digestive discomfort, eczema and alternation between diarrhoea and constipation in addition to typical symptoms of FPIAP (Table 2).
Tolerance and Duration of the GAPS Nutritional Protocol
Five children with FPIES (71.4%) became tolerant to all offending foods between eighteen months and seven years of age. One boy (case 3, now three years old) was not tested against his trigger foods. One girl (case 8, now 14 years old) who initially couldn’t eat anything without reacting, now shows FPIES symptoms only to soy. She became tolerant to all other foods by the age of four years.
For all trigger foods except soy and excluding the boy who was not tested against his culprit foods, the median age of FPIES tolerance of our cohort is 4.4 years. Knowing that the age of the child when the GAPS Nutritional Protocol was implemented was likely to influence the age of recovery, we analysed the duration between the implementation of the GAPS Protocol and recovery. Children of our cohort followed the GAPS Nutritional Protocol for a period of 11 to 36 months before becoming tolerant to their FPIES trigger foods (except soy for one child), with a median duration of 24.5 months.
The FPIAP participant remained on the GAPS Nutritional Protocol for six months before reaching tolerance to all foods by the age of one year.
Breastfeeding and Vaccinations
The eight children of our cohort have all been breastfed for a period of 12 weeks to 4 years (Table 3). We chose not to calculate the median breastfeeding duration, as two children are still being breastfed. More than half of the parents of children with FPIES (57.1%) drew a relationship between vaccinating their child and enterocolitis onset.
Table 1. Demographics and food triggers.
Those applying to at least half of the cohort are in bold.
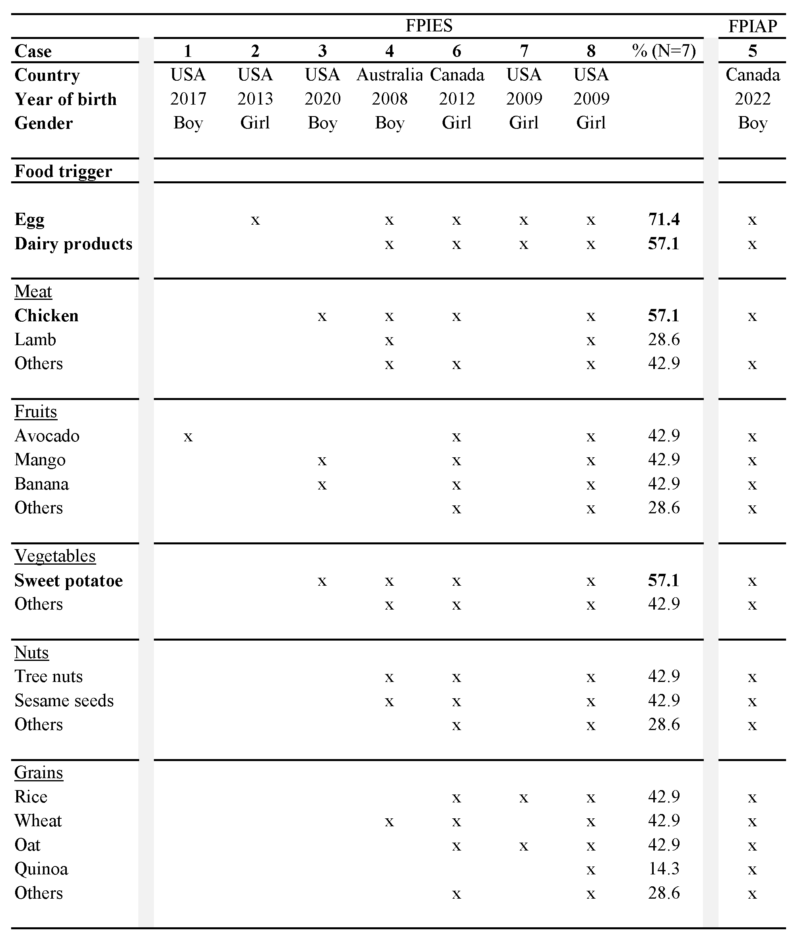
Figure 1. Offending foods in the FPIES cohort (N=7).
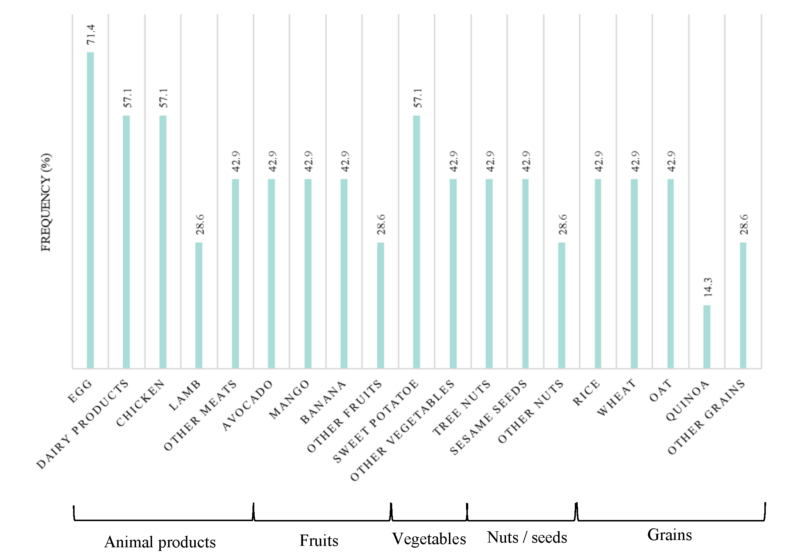
Table 2. Summary of symptoms for each patient in the cohort.
Symptoms affecting at least half the cohort are in bold.
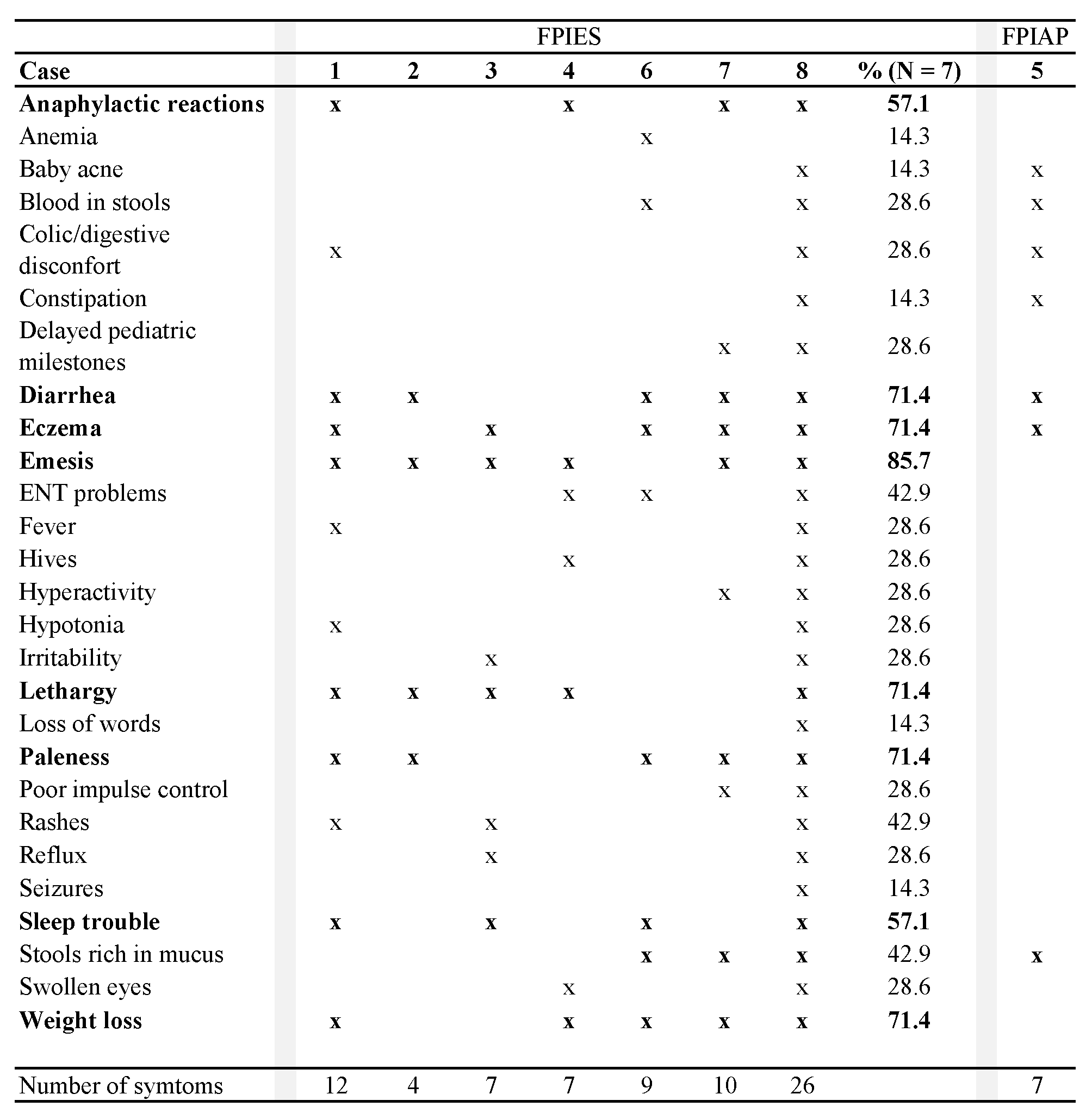
Table 3. GAPS diet duration (in months, except when mentioned) to achieve food tolerance, in relationship with breastfeeding duration and vaccinations. Age of tolerance is the age in months of the child when they became tolerant to their trigger foods.
Discussion
Food Protein-Induced Enterocolitis Syndrome
Parents of FPIES children can feel powerless. In a recent study undertaken on a cohort of 441 children with FPIES, 24.6% of children did not attend school or daycare because of FPIES (Maciag et al., 2020). It seems urgent to provide these families with efficient solutions to improve their quality of life.
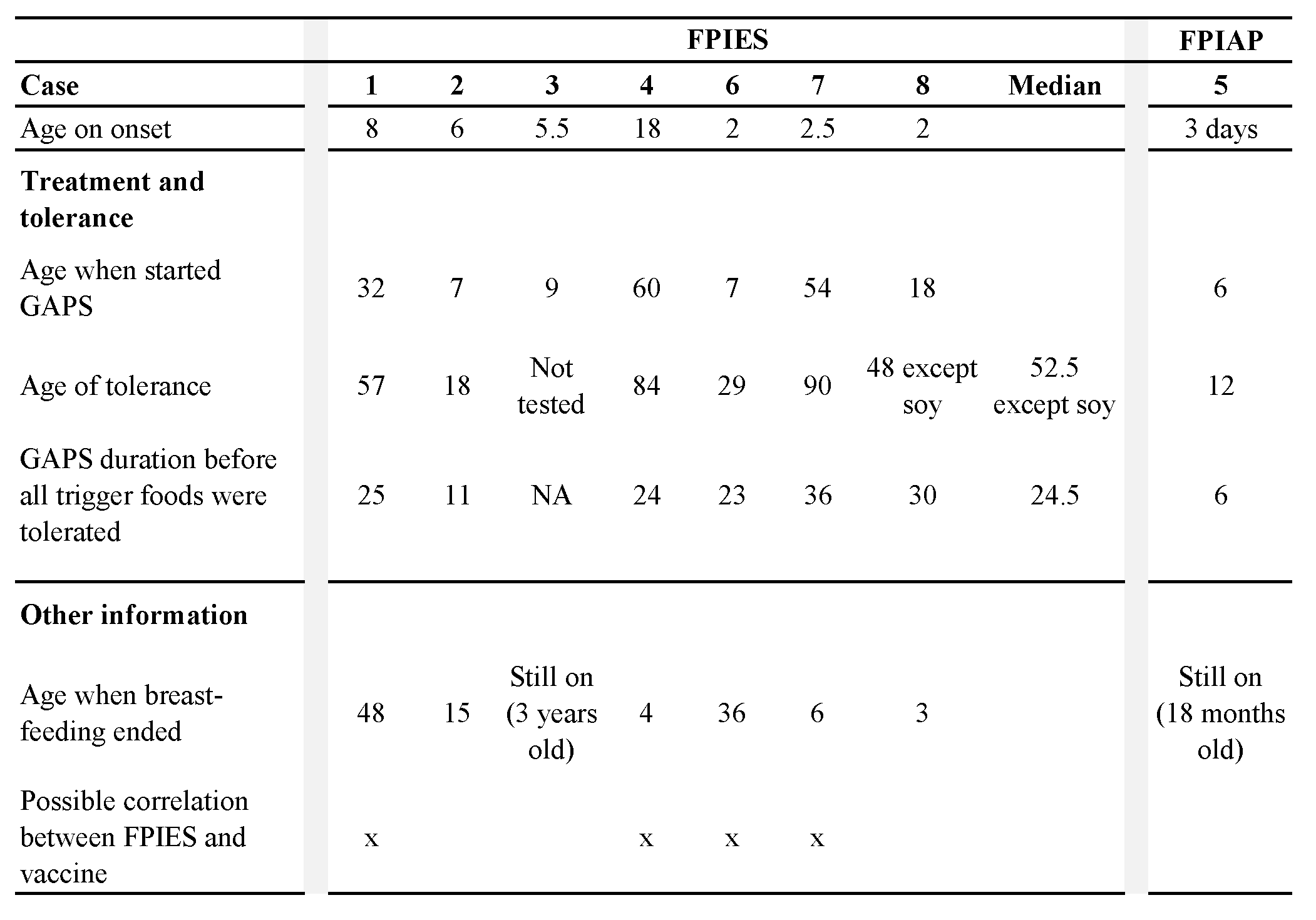
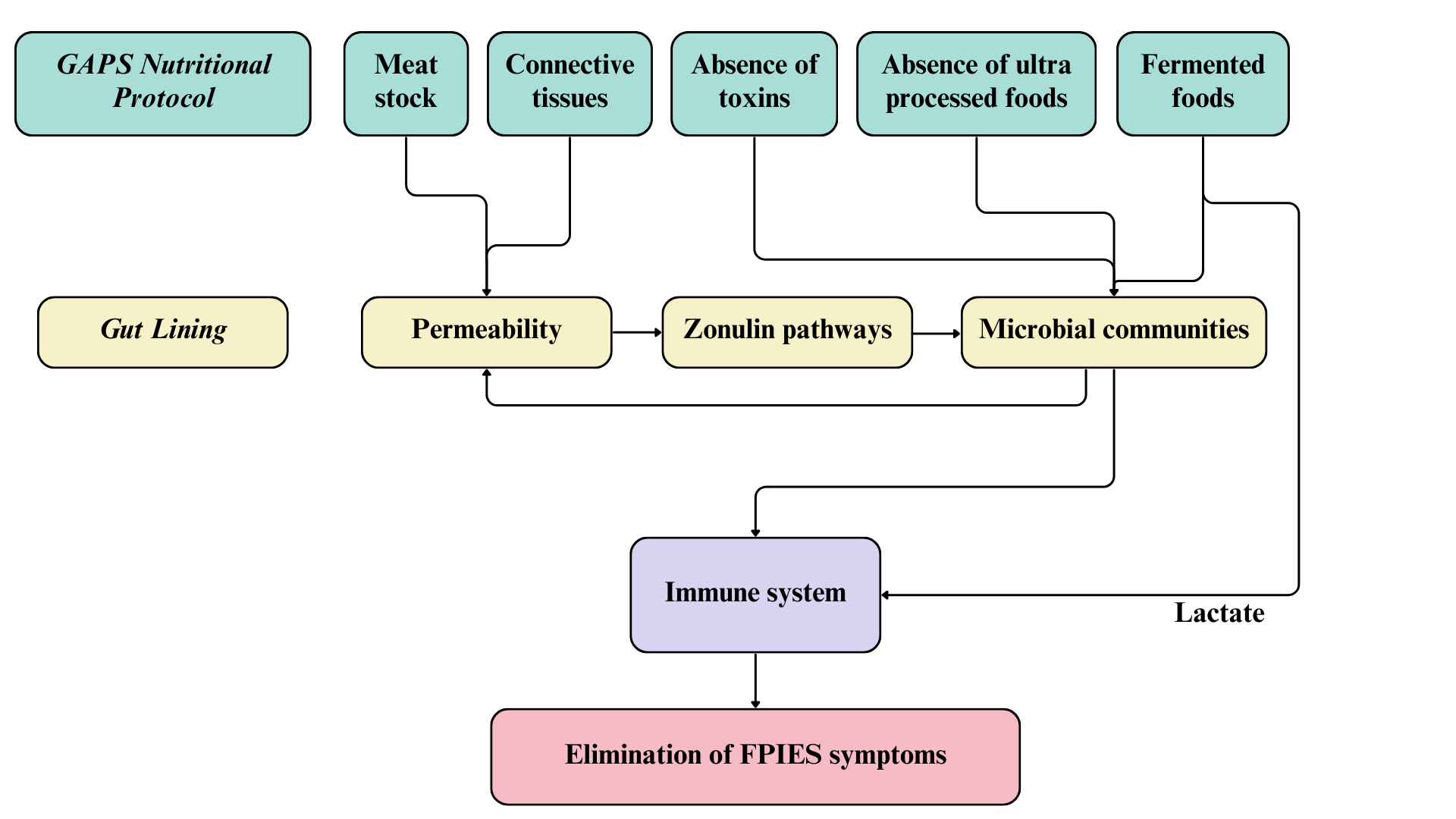
Mainstream recommendations for FPIES involve elimination of trigger foods from the patient’s diet, or from the mother’s diet in case of an exclusively breastfed infant (Nowak-Wegrzyn et al., 2017). This mainstream approach has failed for all children with FPIES in our cohort. The absence of success with the mainstream strategy is due to the lack of understanding of the root cause of FPIES. Following the concept of GAPS (Gut And Psychology Syndrome / Gut And Physiology Syndrome), FPIES is the result of a damaged intestinal lining that became porous and leaky (Campbell-McBride, 2010, 2020). Intestinal permeability causes a disorder in the zonulin pathway, which affects movements of molecules between bloodstream and intestinal lumen and reduces efficiency in the regulation of microbial communities in the proximal colon, causing many diseases related to the immune system (Fasano, 2012, 2020).
This shows that immune disorders are not auto-perpetuating mechanisms, as they can be fixed by restoring intestinal barrier functions (Fasano, 2012).
First, the GAPS Diet excludes ultra processed foods whose regular consumption affects the composition of intestinal microbial communities and creates gut inflammation, disrupting intestinal natural functions (Cuevas-Sierra, Milagro, Aranaz, Martínez, & Riezu-Boj, 2021; Zinöcker & Lindseth, 2018). The GAPS Nutritional Protocol also reduces exposure to toxins by removing chemical products from house cleaning, personal care products, food products and household equipment (Campbell-McBride, 2010, 2020). It has been proven, for example, that pesticides cause disorders in the gut microbiota, by disrupting its composition and functionality and by causing an increase of microbial strains involved in the process of detoxification (Giambò, Teodoro, Costa, & Fenga, 2021). The non-consumption of ultra processed foods and the non-exposure to toxins therefore eliminate the causes of disruption in the gut microbiome, authorizing the gastrointestinal and immune functions to be restored thanks to the nourishing foods found in the GAPS Diet.
The GAPS Nutritional Protocol heals and seals the intestinal wall, as the GAPS Diet provides concentrated nutrition and the right environment to rebuild the gut lining anew. Once the ‘leak’ stops, food starts digesting properly before it is absorbed. As a result, food allergies and intolerances disappear (Campbell-McBride, 2010, 2020). In their search to heal their children from FPIES, the parents in our cohort tried the GAPS Nutritional Protocol with success.
Children of our cohort followed the GAPS Nutritional Protocol for six months to three years before becoming tolerant to all their offending foods, except soy for one child. All children in our cohort implemented the GAPS Introduction Diet, staying on stages one and two for long periods of time. In these stages the diet is composed of meat stock made with gelatinous meats, organ meats, animal fat, cooked low-fibre vegetables and fermented foods (Campbell-McBride, 2010, 2020). These stages of the GAPS Introduction Diet provide maximum healing for a damaged and leaky intestinal wall, leading to seal and heal gut lining within two to three years (Campbell-McBride, 2010, 2020). Indeed, in our cohort this was confirmed. One child started the GAPS protocol with the No-Plant GAPS Diet, which has been created for patients with particularly sensitive gut (Campbell-McBride, 2020). The No-Plant GAPS Diet includes only animal foods; all plant matter is excluded. This form of the GAPS Diet is the gentlest for the gut lining and very healing. After enough healing is achieved in the gut on the No-Plant GAPS Diet, the person moves into the first stage of the GAPS Introduction Diet (Campbell-McBride, 2020).
Meat stock is a staple in the GAPS Diet because it provides gelatin, amino acids, fat-soluble vitamins, minerals and all other elements necessary to reduce bowel inflammation (Busari, Bello, Daramola, & Lajide, 2019). Intestinal mucosa contains connective tissue that plays a crucial role in proliferation and differentiation of intestinal epithelial cells (Riecken et al., 1989), and therefore acts as a key feature in intestinal barrier functions. Meat stock is made by cooking connective tissues of animals in unchlorinated water, creating a healing remedy for the damaged intestinal wall of our patients. The more meat stock GAPS patients consume daily, the quicker they heal (Campbell-McBride, 2010, 2020).
Not only are the intestines restored to health, but so is all other connective tissues in the body. The GAPS Diet contains a high amount of animal products made of connective tissues, such as joints, bones, skin and organs (Campbell-McBride, 2020). These foods are rich in collagen, a protein that provides peptides known for their anti-inflammatory action and their role in repairing the gastrointestinal tract, in addition to their regulatory role of intestinal flora (Graham, Drucker, Diegelmann, & Elson, 1987; Xing et al., 2022). Xing and collaborators (2022) found healthier intestinal epithelium cells and mucosa after treatment with gelatine peptides. It is well recognized that patients with FPIES have deteriorated intestinal mucosa and increased gastrointestinal permeability, with reduced thickness of the small intestinal mucosa, pneumatosis intestinalis or Ileus (Baker, Berin, & Sicherer, 2022; Fontaine & Navarro, 1975; Kim et al., 2022; Lu & Zhang, 2021). In addition, intestinal epithelial cells play a crucial role in the immune balance and protection of the host against pathogens (Peterson & Artis, 2014). The GAPS Diet, rich in collagen and other necessary elements for rebuilding connective tissues, not only heals the intestinal lining, but also helps restore a healthy immune system in FPIES patients.
Fermented foods are an important part of the GAPS Nutritional Protocol. Raw organic milk is fermented at home for 24 hours in order to remove lactose and pre-digest proteins. Homemade yoghurt, kefir, sour cream, whey and cottage cheese are an important part of the GAPS Diet providing active probiotic microbes, enzymes and highly bioavailable nutrients. Fermented vegetables are made at home using lactic-acid fermentation and consumed daily, providing probiotic microbes, bioavailable vitamin C and enzymes (Campbell-McBride, 2010, 2020). Fermented products introduce a high amount and a good diversity of probiotic yeast and bacteria into the gastrointestinal tract (Dimidi, Cox, Rossi, & Whelan, 2019), that survive during their transit through the intestines (Larsen et al., 2011; Wieërs et al., 2020) and are later found in faecal samples (Larsen et al., 2011; Toscano, De Grandi, Miniello, Mattina, & Drago, 2017; Yilmaz et al., 2017). Some researchers found an insignificant change in intestinal bacterial diversity or composition following the intake of probiotic microorganisms (Larsen et al., 2011; Wieërs et al., 2020). They explained this result by the small size of their cohort or by the difference between the bacterial composition of stools used to analyse the intestinal microbiota and the actual microbial composition of the intestinal mucosa (Eckburg et al., 2005; Mentula et al., 2005). Nevertheless, the increase of total bacteria and decrease of pathogenic bacteria levels in faecal samples, following ingestion of milk kefir strains, suggest a change in the composition of intestinal microbiota (Jeong et al., 2017; Kim, Jeong, Kim, & Seo, 2019).
The diversification and the enrichment of the intestinal microbiota with fermented foods in the GAPS Diet may play a significant role in the process of healing from FPIES, through their action on the immune system. It is well established that the FPIES pathophysiology results from an abnormal immune reaction (Berin, 2021; Caubet et al., 2017; Vansickle, Powell, McDonald, & Goldblum, 1985). FPIES is associated with a proinflammatory response: inflammatory cytokines such as tumour necrosis factor-alpha (TFN-α) and interleukin (IL)-8 are found in significantly higher concentrations in the blood of subjects with FPIES than in subjects with IgE-mediated diseases or control groups (Berin et al., 2021; Kimura, Shimomura, Morishita, & Meguro, 2017; Morita et al., 2013). Mahapatro et al. (2021) found a positive correlation between the intestinal microbial community and the production of cytokines, small proteins that activate the immune response. Imbalanced microbiota found in patients with FPIES (Boyer & Scuderi, 2017; Su et al., 2023) may thus cause an elevation of cytokine concentrations that disrupt the immune system. This effect can be thwarted by the consumption of probiotics that modify intestinal microbiota and restore balance in the immune activity (Gorbach, 2000), for example, through the anti-inflammatory action of the microbial communities found in fermented dairy products. Through their action on the intestinal microbiota, specific probiotics found in yoghurt and milk kefir act on the intestinal mucosa by enhancing its protective immunity against inflammation, particularly in the small intestine (Maldonado Galdeano, Novotny Nunez, Carmuega, De Moreno De LeBlanc, & Perdigon, 2015; Perdigon, De Moreno de LeBlanc, Valdez, & Rachid, 2002; Spencer et al., 2022; Vinderola, Perdigón, Duarte, Farnworth, & Matar, 2006). As a result, the consumption of these fermented foods may reduce the inflammatory response in the intestine and reduce FPIES symptoms in case of ingestion of the trigger food.
As FPIES is not an IgE-mediated process, researchers have looked for antigen-specific T-cells to try to understand the mechanism that induce FPIES symptoms from a specific food trigger (Nowak-Wegrzyn et al., 2020). Research failed however in identifying the antigen response involved in the FPIES reaction, authors assuming that pathogenic antigen-responsive T-cells are located in the gastrointestinal tract and are undetectable in the peripheral blood used for testing (Berin et al., 2021; Goswami et al., 2017). The increase of IL-2 levels in patients with FPIES nevertheless suggests T-cell activation during an FPIES crisis (Berin et al., 2021; Kimura, Ito, et al., 2017). Additionally, Spencer et al. (2022) showed that lactate, a metabolite resulting from lactic acid fermentation, stimulates the immune response by increasing the number of regulatory T-cells that inhibit T-cell proliferation. As a result, the presence of fermented foods rich in lactate in the GAPS Diet may limit T-cell activation and play a role in the prevention of inappropriate immune response in case of ingestion of offending food, thus reducing symptoms.
Figure 2. Mode of action and implications for treatment of FPIES with the GAPS Nutritional Protocol.
Food Protein-Induced Allergic Proctocolitis
The pathophysiology of FPIAP remains very poorly understood (Nowak-Wegrzyn et al., 2015). Mennini et al. (2020) suggest that FPIAP is correlated with the dysfunction of the intestinal barrier. The eosinophils concentrate in different parts of the gut lining (Lake, 2000), while they would normally spread out almost along the whole digestive tract (Mennini et al., 2020). This suggests the action of the immune system in the mechanism involved in FPIAP. Eosinophils are associated with the regulation process of bacterial communities in the mucus layer located over intestinal epithelial cells, thus possibly affecting the intestinal barrier function (Singh, Brass, Knight, & Cruickshank, 2019). Their role is nevertheless not well defined, as both pro-inflammatory and anti-inflammatory actions of eosinophils have been highlighted during infections (Ondari, Calvino-Sanles, First, & Gestal, 2021; Woodruff, Masterson, Fillon, Robinson, & Furuta, 2011). Karatas et al. (2022) found higher risks of FPIAP in children born from mothers with low consumption of fermented foods during pregnancy and from mothers with vaginal delivery. As ingested bacteria are later found in the gastrointestinal tract (Gorbach, 2000), mothers consuming fermented foods during pregnancy may have a higher gut microbiota diversity. In addition, Bifidobacteria strains found in the gut and vagina share origins (Freitas & Hill, 2018), signifying possible similarities between gut and vaginal microbiota. Moreover, there is good evidence that vaginal flora are transmitted via vaginal delivery to the mother’s offspring (Dominguez-Bello et al., 2010). This suggests a possible relationship between an infant’s gut microbiota and risk of FPIAP. Fermented foods consumed daily in the GAPS Diet thus enhance the diversity of gut microbial communities and very likely contribute to the disappearance of FPIAP by their positive effects on immune regulation. As we have shown for FPIES, other foods of the GAPS Diet such as meat stock and organ meats contribute to strengthening the immune system, which very likely reinforces the efficiency of the protocol to heal FPIAP. Finally, as we have shown above for FPIES, the absence of ultra processed foods from the GAPS Diet and the elimination of toxins may also contribute to its success in healing eosinophilic proctocolitis. The removal of these offending foods from the infant’s diet enables the intestine to heal and the immune system to rebalance (Campbell-McBride, 2020).
Comorbidities
In addition to the common FPIES or FPIAP symptoms, a high proportion of our cohort experienced skin problems, anaphylactic reactions, weight loss, sleep issues and/or psychological conditions. These symptoms are frequent in patients with FPIES (Mattingly, Mukkada, Smith, & Pitts, 2015; Mehr, Frith, Barnes, & Campbell, 2017; Nowak-Wegrzyn et al., 2019; Su et al., 2020). According to the GAPS theoretical background, and the growing amount of literature on the gut-health axis, these comorbidities also stem from damaged intestines and an imbalanced immune system (Campbell-McBride, 2010, 2020). This can explain why, in our study, all collateral symptoms were resolved when implementing the GAPS Nutritional Protocol.
Skin disorders, especially eczema, are fairly common in FPIES (Nowak-Węgrzyn, 2015), although Lake (2000) reported rare eczema in FPIAP. Despite some previous doubts about the effects of probiotic intake on the reduction of skin problems (Larsen et al., 2011), the positive effect of probiotic intake on these skin problems has recently been reported (Jeong et al., 2017; Yu, Dunaway, Champer, Kim, & Alikhan, 2020). The GAPS Nutritional Protocol provides a high number of probiotic microbes in the form of fermented foods, giving one explanation for the success of the GAPS Diet in clearing skin problems.
Regarding anaphylactic allergies, it has been shown that mechanisms differ from IgE-mediated allergies to non-IgE mediated allergies, with a distinct humoral immune response (Shek et al., 2005). This may explain why patients with FPIES and undetectable IgE-mediated allergies recover faster than patients experiencing both FPIES and IgE-mediated allergies (Caubet et al., 2014). Fixing several malfunctions of the immune system may take longer. The cases we present in this study confirm that the GAPS Nutritional Protocol, with its multiple actions on the intestines and the immune system, can resolve both IgE-mediated and non-IgE mediated allergies. This clinical observation invites researchers to look at the role of the intestines and its microbiome in anaphylaxis.
Weight loss was common in our cohort. We suggest that this is explained by the deterioration of the intestinal lumen found in patients with FPIES (Fontaine & Navarro, 1975; Kim et al., 2022; Lu & Zhang, 2021). Damaged intestines are unable to digest and absorb food, leading to nutritional deficiencies and weight loss (Campbell-McBride, 2020). All children in our cohort who lost weight before the GAPS Nutritional Protocol started putting on weight soon after starting the GAPS Diet, demonstrating that the intestines started healing and digesting food appropriately.
Sleep disturbance is another common symptom of FPIES, experienced by 57.1 % of our cohort. Evidence of a correlation between sleep quality and gut microbiota has been reported, explaining why patients suffering from sleep disturbance also experience digestive symptoms (Han, Yuan, & Zhang, 2022). Sleep physiology highly depends on the composition of intestinal bacterial communities, with modulation of gut microbiota improving sleep quality (Sen et al., 2021). This explains why the implementation of the GAPS diet, rich in probiotic foods, resolved sleep disorders in our FPIES cohort.
Finally, certain children of our cohort experienced cognitive and developmental symptoms such as delayed paediatric milestones, loss of words, irritability or hyperactivity. These resolved with the implementation of the GAPS Nutritional Protocol. The brain-gut-microbiome axis has been the core of many research projects, and it is now well established that any disturbance in the loop linking the brain to the intestines and to the gut microbiota can affect the other features of the circle (Martin, Osadchiy, Kalani, & Mayer, 2018). For instance, the presence of specific bacteria in the gut microbiota (Wang et al., 2020), and a higher frequency of digestive symptoms in children with Attention‑deficit/hyperactivity disorder (ADHD), suggest the implication of gut dysbiosis in the mechanism involved in ADHD (Ming et al., 2018). The success of the GAPS Diet in healing neurodevelopmental disorders such as ADHD can thus be explained by its ability to rebalance gut microbiota and restore a healthy gut lumen (Campbell-McBride, 2010, 2020).
Tolerance
In our study, the majority of the FPIES cohort (71.4%) reacted to at least four foods, which is very rare, as prevalence of children with multiple FPIES is evaluated to less than 10% (Metbulut et al., 2022; Nowak-Wegrzyn et al., 2020). Nowak-Wegrzyn and collaborators (2020) suggest the existence of a “more persistent phenotype” (p.26), associated with severe cases including FPIES to multiple foods. They explain that people with this phenotype may become tolerant to offending food only at adult age. Our results show that the GAPS Nutritional Protocol helps reach tolerance earlier, as 80% of our cohort with more than four FPIES triggers reached full tolerance before the age of seven and a half years, in eleven months to three years. Nowak Wegrzyn and colleagues (2017) report the possibility of FPIES persistence until adult age for FPIES induced by cow’s milk or soy, whereas other trigger foods are commonly tolerated between the ages of three and five years. The GAPS Nutritional Protocol shows high efficiency, as the two children who showed FPIES reactions to a single food (egg or avocado), recovered after a period of 11 and 25 months respectively, before the age of two and five years.
Recovery however depends on many factors, such as culprit food and country (Nowak-Wegrzyn et al., 2017). In an American study (Caubet et al., 2014), FPIES children became tolerant at a median age respectively of 13.8, 6.7, 4.7 and 4.0 years for milk, soy, rice and oats, showing an influence of trigger food on age of tolerance. Recovery rates also vary with the country. In Israel, Katz et al. (2011) reported a median age of tolerance lower than one year for cow’s milk in a birth cohort. Similar results have been observed for cow’s milk in Japan and Turkey (Kimura, Shimomura, et al., 2017; Metbulut et al., 2022). In Greece and the United States, lower recovery rates have been obtained for milk with a median age of tolerance of respectively two years (Douros et al., 2019) and 13.8 years (Caubet et al., 2014). This difference in findings could be due to a difference in methodology, with the inclusion or exclusion of patients with milder symptoms, the inclusion of patients with different trigger foods, or different criteria used to identify the recovery age (oral food challenge or offending food consumed at home).
Despite changes in consumption patterns over the last decades, variability in the composition of conventional diets with country (Kromhout et al., 1989) may also explain why recovery may take longer in some countries. As FPIES is related to intestinal health, the consumption of refined sugar and processed foods keeps nourishing intestinal pathogenic bacteria and yeasts, deteriorating the microbiota and weakening the immune system (Campbell-McBride, 2010, 2020). This may prevent patients with FPIES from recovery despite the avoidance of the trigger food.
In addition, microbial composition found in fermented foods differ between Western and Eastern countries (Tamang et al., 2020). Knowing the effect of probiotics on the gut lumen, intestinal permeability and immune system (Gorbach, 2000), this could be another reason to explain variability between countries in the ability to recover from FPIES. The solution implemented to deal with FPIES may also play a crucial role in the recovery process and the age at which it occurs. The two American children of our cohort with cow’s milk-induced FPIES became tolerant to dairy products by the age of four years and a half (case 7) and four years (case 8), which is much lower than the median of 13.8 years found by Caubet and collaborators in the USA (2014). However, despite spectacular progress in her healing process, subject 8 still experiences FPIES reactions with soy (not allowed in the GAPS diet) at 14 years old, which is older than the median age of 6.7 years found by Caubet and collaborators (2014). Finally, recovery age obviously depends on the age of the first FPIES reaction and on the age of the child when implementing a solution (elimination diet, GAPS Protocol or other). Logically, the sooner the problem is considered, the earlier the child has a chance to heal.
Variability of recovery age caused by these multiple factors make comparisons among patients within studies and between studies quite complex and proves the uniqueness of each person in healing FPIES.
Breastfeeding
Like other authors (Caubet et al., 2014; Katz & Goldberg, 2014; Lake, 2000; Mehr et al., 2017), we found no positive effect of exclusive breastfeeding on the onset of FPIES or FPIAP, although others found rare occurrence of FPIES in exclusively breastfed children (Nowak-Węgrzyn, 2015). Our results do not support the hypothesis of Mehr and colleagues (2017) that long and exclusive lactation tends to prevent FPIES to multiple foods, as subjects 3 and 6 were breastfed until at least three years of age and yet experienced FPIES to multiple trigger foods. This can be explained by the origins of the intestinal flora composition. We have discussed above how babies with FPIES or FPIAP have a dysfunctional immune system that results from pathogenic gut microbiota. Babies born via vaginal birth inherit their microbial diversity from their mother’s vagina (Dominguez-Bello et al., 2010). As a result, babies born naturally from a mother with an imbalanced vaginal microbiota acquire their mother’s dysfunctional microbial diversity at birth. With a disrupted initial bacterial community, the infant is more likely to develop dysbiosis (Walker & Iyengar, 2015) and disorders such as FPIAP and FPIES. This initial bacterial community will be only slightly affected by breast-feeding. Indeed, during lactation, the infant’s intestinal microbiota is inoculated by the mother’s gut bacteria that migrate to breast milk through lymphoid cells (Walker & Iyengar, 2015). These additional bacteria nevertheless represent only a small proportion of the total amount of microbes found in the bacterial community of the infant (Walker & Iyengar, 2015), limiting the role of mother’s milk in enriching her infant’s microbiome. Thus, breast-feeding can hardly influence FPIES or FPIAP onset and duration. However, in addition to being the perfectly adapted nutrition source for an infant, human milk contains active growth factors, hormones and immune cells such as cytokines, immunoglobulins or inflammatory mediators, promoting optimal development of the child and immune protection (Oddy, 2002; Vieira Borba, Sharif, & Shoenfeld, 2018). In that, breast-feeding may limit the damage caused by imbalanced intestinal microbiota on the child’s health, explaining that incidence and duration of exclusive breast-feeding can be significantly lower in infants with multiple trigger foods than in children suffering from a single proctocolitis trigger (Buyuktiryaki et al., 2020).
Vaccination
More than half of the mothers of the patients in our cohort drew a relationship between FPIES onset and childhood vaccinations. As demonstrated above, FPIES is related to immune imbalance. As vaccination affects the immune system and activates it to encourage the body to react to a specific microbe, it directly disturbs immune balance (Siegrist, 2008). The immune system of people with FPIES may strongly react to vaccination due to its initial state of disequilibrium and fragility, causing the onset of symptoms never experienced before.
Limitations
Sometimes seen as a limited methodology with biases due to selection criteria and differences in treatment, retrospective research contains the wealth of historical records that are powerful to understand rare diseases and that serve as a foundation for future prospective studies (Talari & Goyal, 2020). Further research is now required to compare the efficiency of the GAPS Nutritional Protocol on FPIES and FPIAP with the efficiency of the conventional elimination diet, on a larger cohort.
Conclusion
Eight case studies, presented here, show that the GAPS Nutritional Protocol is a promising treatment for children with FPIES or FPIAP. The GAPS Nutritional Protocol helped children to heal with high efficiency, even in cases with multiple trigger foods, where participants had failed to see any improvement with the conventional elimination diet. The GAPS Nutritional Protocol eliminates ultra processed foods and toxicity, in addition to starch and grains, offering the possibility for the restoration of intestinal functions. In addition, it is rich in all nutrients required by the gut and the immune system to heal and fulfill their natural functions.
A strong point of the GAPS Diet resides in the high consumption of fermented foods rich in beneficial bacteria and yeasts that rebalance the immune system: 1) by their direct effect through the production of lactate, and 2) by their indirect effects through the diversification of gut microbiota.
Meat stock and animal products rich in connective tissue and collagen are daily consumed in the GAPS Diet and promote the healing process of the gut lining. This reduces permeability of the intestinal mucosa, improves gut barrier functions and enhances the regulation of microbial communities in the colon, in addition to improving immune functions. As a result, FPIES with its side effects such as neurodevelopmental disorders, sleep disorders or skin problems disappear.
FPIAP remains poorly known but is likely to be also correlated to microbiota communities and the immune system. As for FPIES, the GAPS Nutritional Protocol may be beneficial thanks to its diverse actions on the composition of microbial communities and on immune regulation.
The human body is a complex organism occupied by a high diversity of microorganisms such as bacteria, yeast and fungi that live in a fragile balance in the gut microbiome and elsewhere. A toxin-free environment and a natural diet with nourishing foods, such as those provided by the GAPS Nutritional Protocol, are the key elements to restore the natural equilibrium required for optimal intestinal barrier functions and immune regulation, in order to heal non-IgE mediated food allergies like FPIES or FPIAP.
Declarations
This paper was written following the CARE guidelines.
Acknowledgements
We are very grateful to all participants who kindly shared their personal stories with us, although this could bring back painful memories. We acknowledge Nichole Sawatzky and Becky Plotner, Certified GAPS Practitioners, for their help in recruiting participants.
Ethics Approval and Consent to Participate
Not applicable in a retrospective study.
Consent for Publication
Participants accepted anonymous publication of their data in a written informed consent. Copies of consent forms are available for review.
Availability of Data and Materials
The questionnaires and interviews containing the data of this study are available upon request.
Competing Interests
We, authors, declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Dr. Natasha Campbell-McBride is the creator of the GAPS concept and the GAPS Nutritional Protocol.
Funding
No financial support has been received for the work reported.
Authors’ Contributions
Ophélie Planckaert collected and analysed the datasets, designed and wrote the manuscript. Natasha Campbell-McBride supervised and like Stephanie Seneff and Sophie Delaunay-Vagliasindi contributed to the design of the work and reviewed the paper. All authors read and approved the final manuscript.
Author’s Information
Ophélie Planckaert has a combined Bachelor’s Master’s (BS/MSc) degree in agrifood science (ENITAC, Clermont-Ferrand, France) in addition to a Master’s degree in Biological Sciences (Université Laval, QC, Canada). She works as a researcher in non-profit organisations on the relationships between gut health and disease.
Stephanie Seneff is a Senior Research Scientist at Massachusetts Institute of Technology (MIT) in Cambridge, MA, USA. She holds a BS degree from MIT in biology, and a Ph.D. in electrical engineering and computer science also from MIT. Her recent research interests are on the role of nutritional deficiencies and toxic chemicals in disease, with a focus on the mineral sulphur and the herbicide glyphosate.
Sophie Delaunay-Vagliasindi holds two Master’s degrees, in Developmental Psychology from the University of Kent, UK, and in Clinical Psychology from the University of Louvain-la-neuve, Belgium. She is specialising in the impact of gut flora on development and conducting research for non-profit organisations, while working with patients in a clinical setting.
Natasha Campbell-McBride is a medical doctor with two postgraduate degrees: MMedSci in neurology and MMedSci in human nutrition. She is the creator of the GAPS concept and the GAPS Nutritional Protocol, described in detail in her two books Gut And Psychology Syndrome (2nd edition 2010) and Gut And Physiology Syndrome (2020).
Appendix appears below References.
References
Ābele, S., Meija, L., Folkmanis, V., & Tzivian, L. (2021). Specific Carbohydrate Diet (SCD/GAPS) and Dietary Supplements for Children with Autistic Spectrum Disorder. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences., 75(6), 417–425. https://doi.org/10.2478/prolas-2021-0062
Aslan, N., Koca, T., & Akcam, M. (2017). Evaluation of Our Food Protein Induced Proctocolitis Cases: A Single Center Experience. J Clin Gastroenterol Hepatol, 1(3), 25–28. https://doi.org/10.21767/2575-7733.1000025
Baker, M. G., Berin, M. C., & Sicherer, S. (2022). Update on food protein–induced enterocolitis syndrome (FPIES). Current Allergy and Asthma Reports, 22(10), 113–122. https://doi.org/10.1007/s11882-022-01037-y
Berin, M. C. (2021). Advances in understanding immune mechanisms of food protein–induced enterocolitis syndrome. Annals of Allergy, Asthma & Immunology, 126(5), 478–481. https://doi.org/10.1016/j.anai.2021.01.033
Berin, M. C., Lozano-Ojalvo, D., Agashe, C., Baker, M. G., Bird, J. A., & Nowak-Wegrzyn, A. (2021). Acute FPIES reactions are associated with an IL-17 inflammatory signature. Journal of Allergy and Clinical Immunology, 148(3), 895-901.e6. https://doi.org/10.1016/j.jaci.2021.04.012
Boyer, J., & Scuderi, V. (2017). P504 Comparison of the gut microbiome between food protein-induced enterocolitis sydrome (FPIES) infants and allergy-free infants. Annals of Allergy, Asthma & Immunology, 119(5), e3. https://doi.org/10.1016/j.anai.2017.09.070
Busari, Y. O., Bello, L. A., Daramola, O. E., & Lajide, L. (2019). Proximate composition and mineral analysis of Goat’s Liver, Cow’s pancreas and their meat stock. International Journal of Recent Innovation in Food Science & Nutrition, 2, 12–20.
Buyuktiryaki, B., Celik, I. K., Erdem, S. B., Capanoglu, M., Civelek, E., Guc, B. U., … Akcal, O. (2020). Risk factors influencing tolerance and clinical features of food protein–induced allergic proctocolitis. Journal of Pediatric Gastroenterology and Nutrition, 70(5), 574–579. https://doi.org/10.1097/MPG.0000000000002629
Campbell-McBride, N. (2010). Gut and Psychology Syndrome: Natural Treatment for Autism, Dyspraxia, A.D.D., Dyslexia, A.D.H.D., Depression, Schizophrenia, 2nd Edition. Medinform Publishing; Revised & enlarged edition.
Campbell-McBride, N. (2020). Gut and Physiology Syndrome: Natural Treatment for Allergies, Autoimmune Illness, Arthritis, Gut Problems, Fatigue, Hormonal Problems, Neurological Disease and More. Medinform Publishing.
Caubet, J. C., Bencharitiwong, R., Ross, A., Sampson, H. A., Berin, M. C., & Nowak-Wegrzyn, A. (2017). Humoral and cellular responses to casein in patients with food protein-induced enterocolitis to cow’s milk. Journal of Allergy and Clinical Immunology, 139(2), 572–583. https://doi.org/10.1016/j.jaci.2016.02.047
Caubet, J. C., Ford, L. S., Sickles, L., Jaervinen, K. M., Sicherer, S. H., Sampson, H. A., & Nowak-Wegrzyn, A. (2014). Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. Journal of Allergy and Clinical Immunology, 134(2), 382-+. https://doi.org/10.1016/j.jaci.2014.04.008
Cetinkaya, P. G., Ocak, M., Sahiner, U. M., Sekerel, B. E., & Soyer, O. (2021). Food protein–induced allergic proctocolitis may have distinct phenotypes. Annals of Allergy, Asthma & Immunology, 126(1), 75–82. https://doi.org/10.1016/j.anai.2020.08.021
Coates, R. W., Weaver, K. R., Lloyd, R., Ceccacci, N., & Greenberg, M. R. (2011). Food protein-induced enterocolitis syndrome as a cause for infant hypotension. Western Journal of Emergency Medicine, 12(4), 512. https://doi.org/10.5811/westjem.2011.2.2134
Cuevas-Sierra, A., Milagro, F. I., Aranaz, P., Martínez, J. A., & Riezu-Boj, J. I. (2021). Gut microbiota differences according to ultra-processed food consumption in a Spanish population. Nutrients, 13(8), 2710. https://doi.org/10.3390/nu13082710
Delahaye, C., Chauveau, A., Kiefer, S., & Dumond, P. (2017). Food protein-induced enterocolitis syndrome (FPIES) in 14 children. Archives de Pediatrie, 24(4), 310–316. https://doi.org/10.1016/j.arcped.2017.01.011
Delaunay-Vagliasindi, S., Seneff, S., & Campbell-McBride, N. (2021). GAPS Nutritional Protocol: How healing the gut removes the basis for all chronic diseases. Journal of Orthomolecular Medicine, 36(3).
Delaunay-Vagliasindi, S., Seneff, S., Coro, S., & Campbell-McBride, N. (2021). GAPS Nutritional Protocol as a Treatment for PANDAS: A Case Study. Journal of Orthomolecular Medicine, 36(3).
Delaunay-Vagliasindi, S., Seneff, S., Coro, S., Plotner, B., & Campbell-McBride, N. (2022). Why the GAPS Nutritional Protocol is a Promising Treatment for Tics and Tic-Related Disorders: Analysis of Six Case Studies. Journal of Orthomolecular Medicine, 37(1).
Dimidi, E., Cox, S. R., Rossi, M., & Whelan, K. (2019). Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients, 11(8). https://doi.org/10.3390/nu11081806
Dominguez-Bello, M. G., Costello, E. K., Contreras, M., Magris, M., Hidalgo, G., Fierer, N., & Knight, R. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences, 107(26), 11971–11975. https://doi.org/10.1073/pnas.1002601107
Douros, K., Tsabouri, S., Feketea, G., Grammeniatis, V., Koliofoti, E. G., Papadopoulos, M., … Priftis, K. N. (2019). Retrospective study identified fish and milk as the main culprits in cases of food protein-induced enterocolitis syndrome. Acta Paediatrica, 108(10), 1901–1904. https://doi.org/10.1111/apa.14779
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., … Relman, D. A. (2005). Diversity of the human intestinal microbial flora. Science, 308(5728), 1635–1638. https://doi.org/10.1126/science.1110591
Fasano, A. (2012). Leaky gut and autoimmune diseases. Clinical Reviews in Allergy & Immunology, 42, 71–78. https://doi.org/10.1007/s12016-011-8291-x
Fasano, A. (2020). All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research, 9, F1000 Faculty Rev-69. https://doi.org/10.12688/f1000research.20510.1
Fontaine, J. L., & Navarro, J. (1975). Small intestinal biopsy in cow’s milk protein allergy in infancy. Archives of Disease in Childhood, 50, 357–362. https://doi.org/10.1136/adc.50.5.357
Freitas, A. C., & Hill, J. E. (2018). Bifidobacteria isolated from vaginal and gut microbiomes are indistinguishable by comparative genomics. PLoS One, 13(4), e0196290.
Giambò, F., Teodoro, M., Costa, C., & Fenga, C. (2021). Toxicology and microbiota: How do pesticides influence gut microbiota? A review. International Journal of Environmental Research and Public Health, 18(11), 5510. https://doi.org/10.3390/ijerph18115510
Gorbach, S. L. (2000). Probiotics and gastrointestinal health. The American Journal of Gastroenterology, 95(1, Supplement 1), S2–S4. https://doi.org/10.1016/S0002-9270(99)00806-0
Goswami, R., Blazquez, A. B., Kosoy, R., Rahman, A., Nowak-Wegrzyn, A., & Berin, M. C. (2017). Systemic innate immune activation in food protein-induced enterocolitis syndrome. Journal of Allergy and Clinical Immunology, 139(6), 1885-+. https://doi.org/10.1016/j.jaci.2016.12.971
Graham, M. F., Drucker, D. E., Diegelmann, R. F., & Elson, C. O. (1987). Collagen synthesis by human intestinal smooth muscle cells in culture. Gastroenterology, 92(2), 400–405. https://doi.org/10.1016/0016-5085(87)90134-x
Gryboski, J. D. (1967). Gastrointestinal milk allergy in infants. Pediatrics, 40(3), 354–362. https://doi.org/10.1542/peds.40.3.354
Halbrich, M., Ben-Shoshan, M., & Rex, G. (2014). Friend or foe? Figuring out the difference between FPIES, IgE-mediated allergy and food intolerance. BMJ Case Reports. https://doi.org/10.1136/bcr-2013-200254
Han, M., Yuan, S., & Zhang, J. (2022). The interplay between sleep and gut microbiota. Brain Research Bulletin, 180, 131–146. https://doi.org/10.1016/j.brainresbull.2021.12.016
Jeong, D., Kim, D.-H., Kang, I.-B., Kim, H., Song, K.-Y., Kim, H.-S., & Seo, K.-H. (2017). Modulation of gut microbiota and increase in fecal water content in mice induced by administration of Lactobacillus kefiranofaciens DN1. Food & Function, 8(2), 680–686.
Karatas, P., Uysal, P., Kahraman Berberoglu, B., Erge, D., & Calisir, H. (2022). The low maternal consumption of homemade fermented foods in pregnancy is an additional risk factor for food protein-induced allergic proctocolitis: A case-control study. International Archives of Allergy and Immunology, 183(3), 262–270. https://doi.org/10.1159/000519154
Katz, Y., & Goldberg, M. R. (2014). Natural history of food protein-induced enterocolitis syndrome. CURRENT OPINION IN ALLERGY AND CLINICAL IMMUNOLOGY, 14(3), 229–239. https://doi.org/10.1097/ACI.0000000000000053
Katz, Y., Goldberg, M. R., Rajuan, N., Cohen, A., & Leshno, M. (2011). The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: A large-scale, prospective population-based study. Journal of Allergy and Clinical Immunology, 127(3), 647-U163. https://doi.org/10.1016/j.jaci.2010.12.1105
Kim, D. H., Jeong, D., Kim, H., & Seo, K.-H. (2019). Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Critical Reviews in Food Science and Nutrition, 59(11), 1782–1793. https://doi.org/10.1080/10408398.2018.1428168
Kim, Y. L., Joo, J. Y., Jung, Y. H., Choi, C. W., Kim, B. I., & Yang, H. R. (2022). Differentiation of food protein-induced enterocolitis syndrome misleading to necrotizing enterocolitis. Annals of Allergy, Asthma & Immunology, 128(2), 193–198. https://doi.org/10.1016/j.anai.2021.09.024
Kimura, M., Ito, Y., Shimomura, M., Morishita, H., Meguro, T., Adachi, Y., & Seto, S. (2017). Cytokine profile after oral food challenge in infants with food protein-induced enterocolitis syndrome. Allergology International, 66(3), 452–457. https://doi.org/10.1016/j.alit.2016.12.001
Kimura, M., Shimomura, M., Morishita, H., & Meguro, T. (2017). Prognosis of infantile food protein-induced enterocolitis syndrome in Japan. Pediatrics International, 59(8), 855–860. https://doi.org/10.1111/ped.13305
Kromhout, D., Keys, A., Aravanis, C., Buzina, R., Fidanza, F., Giampaoli, S., … Pekkarinen, M. (1989). Food consumption patterns in the 1960s in seven countries. The American Journal of Clinical Nutrition, 49(5), 889–894.
Lake, A. M. (2000). Food-induced eosinophilic proctocolitis. JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION, 30, S58–S60. https://doi.org/10.1097/00005176-200001001-00009
Larsen, N., Vogensen, F. K., Gøbel, R., Michaelsen, K. F., Al-Soud, W. A., Sørensen, S. J., … Jakobsen, M. (2011). Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp. Lactis Bi-07. FEMS Microbiology Ecology, 75(3), 482–496. https://doi.org/10.1111/j.1574-6941.2010.01024.x
Lemoine, A., Colas, A. S., Le, S., Delacourt, C., Tounian, P., & Lezmi, G. (2022). Food protein‐induced enterocolitis syndrome: A large French multicentric experience. Clinical and Translational Allergy, 12(2). https://doi.org/10.1002/clt2.12112
Lu, Y., & Zhang, Z.-Q. (2021). Food protein-induced enterocolitis syndrome presenting after necrotizing enterocolitis in a preterm neonate: A case report. Translational Pediatrics, 10(5), 1393. https://doi.org/10.21037/tp-21-9
Maciag, M. C., Bartnikas, L. M., Sicherer, S. H., Herbert, L. J., Young, M. C., Matney, F., … Bingemann, T. A. (2020). A Slice of Food Protein–Induced Enterocolitis Syndrome (FPIES): Insights from 441 Children with FPIES as Provided by Caregivers in the International FPIES Association. The Journal of Allergy and Clinical Immunology: In Practice, 8(5), 1702–1709. https://doi.org/10.1016/j.jaip.2020.01.030
Mahapatro, M., Erkert, L., & Becker, C. (2021). Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut. Cells, 10(1), 111. https://doi.org/10.3390/cells10010111
Maldonado Galdeano, C., Novotny Nunez, I., Carmuega, E., De Moreno De LeBlanc, A., & Perdigon, G. (2015). Role of Probiotics and Functional Foods in Health: Gut Immune Stimulation by Two Probiotic Strains and a Potential Probiotic Yoghurt. ENDOCRINE METABOLIC & IMMUNE DISORDERS-DRUG TARGETS, 15(1), 37–45. https://doi.org/10.2174/1871530314666141216121349
Maloney, J., & Nowak-Wegrzyn, A. (2007). Educational clinical case series for pediatric allergy and immunology: Allergic proctocolitis, food protein-induced enterocolitis syndrome and allergic eosinophilic gastroenteritis with protein-losing gastroenteropathy as manifestations of non-IgE-mediated cow’s milk allergy. Pediatric Allergy and Immunology, 18(4), 360–367. https://doi.org/10.1111/j.1399-3038.2007.00561.x
Martin, C. R., Osadchiy, V., Kalani, A., & Mayer, E. A. (2018). The Brain-Gut-Microbiome Axis. Cellular and Molecular Gastroenterology and Hepatology, 6(2), 133–148. https://doi.org/10.1016/j.jcmgh.2018.04.003
Mattingly, R., Mukkada, V., Smith, A., & Pitts, T. (2015). Optimizing an Aversion Feeding Therapy Protocol for a Child with Food Protein-Induced Enterocolitis Syndrome (FPIES). Journal of Pulmonary & Respiratory Medicine, 5(4), 287. https://doi.org/10.4172/2161-105X.1000287
Mehr, S., Frith, K., Barnes, E. H., & Campbell, D. E. (2017). Food protein-induced enterocolitis syndrome in Australia: A population-based study, 2012-2014. Journal of Allergy and Clinical Immunology, 140(5), 1323–1330. https://doi.org/10.1016/j.jaci.2017.03.027
Mennini, M., Fiocchi, A. G., Cafarotti, A., Montesano, M., Mauro, A., Villa, M. P., & Di Nardo, G. (2020). Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organization Journal, 13(10), 100471. https://doi.org/10.1016/j.waojou.2020.100471
Mentula, S., Harmoinen, J., Heikkilä, M., Westermarck, E., Rautio, M., Huovinen, P., & Könönen, E. (2005). Comparison between cultured small-intestinal and fecal microbiotas in beagle dogs. Applied and Environmental Microbiology, 71(8), 4169–4175. https://doi.org/10.1128/AEM.71.8.4169-4175.2005
Metbulut, A. P., Ozen, S., Kendirci, N., Guc, B. U., Guvenir, H., Vezir, E., … Orhan, F. (2022). Evaluation of the Clinical Characteristics of Patients with Food Protein-Induced Enterocolitis Syndrome: A Multicenter Study. INTERNATIONAL ARCHIVES OF ALLERGY AND IMMUNOLOGY, 183(8), 805–813. https://doi.org/10.1159/000522496
Ming, X., Chen, N., Ray, C., Brewer, G., Kornitzer, J., & Steer, R. A. (2018). A gut feeling: A hypothesis of the role of the microbiome in attention-deficit/hyperactivity disorders. Child Neurology Open, 5, 2329048X18786799. https://doi.org/10.1177/2329048X1878679
Morita, H., Nomura, I., Orihara, K., Yoshida, K., Akasawa, A., Tachimoto, H., … Matsumoto, K. (2013). Antigen-specific T-cell responses in patients with non-IgE-mediated gastrointestinal food allergy are predominantly skewed to T(H)2. JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY, 131(2), 590–592. https://doi.org/10.1016/j.jaci.2012.09.005
Murray, K. F., & Christie, D. L. (1993). Dietary protein intolerance in infants with transient methemoglobinemia and diarrhea. Journal of Pediatrics, 122(1), 90–92. https://doi.org/10.1016/s0022-3476(05)83495-x
Nowak-Węgrzyn, A. (2015). Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy and Asthma Proceedings, 36(3), 172–184. https://doi.org/10.2500/aap.2015.36.3811
Nowak-Wegrzyn, A., Berin, M. C., & Mehr, S. (2020). Food Protein-Induced Enterocolitis Syndrome. The Journal of Allergy and Clinical Immunology: In Practice, 8(1), 24–35. https://doi.org/10.1016/j.jaip.2019.08.020
Nowak-Wegrzyn, A., Chehade, M., Groetch, M. E., Spergel, J. M., Wood, R. A., Allen, K., … Greenhawt, M. (2017). International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. Journal of Allergy and Clinical Immunology, 139(4), 1111-+. https://doi.org/10.1016/j.jaci.2016.12.966
Nowak-Wegrzyn, A., Katz, Y., Mehr, S. S., MBBS, BMedSci, FRCPA, … Koletzko, S. (2015). Non–IgE-mediated gastrointestinal food allergy. Journal of Allergy and Clinical Immunology, 135(5), 1114–1124. https://doi.org/10.1016/j.jaci.2015.03.025
Nowak-Wegrzyn, A., Warren, C. M., Brown-Whitehorn, T., Cianferoni, A., Schultz-Matney, F., & Gupta, R. S. (2019). Food protein–induced enterocolitis syndrome in the US population–based study. Journal of Allergy and Clinical Immunology. https://doi.org/10.1016/j.jaci.2019.06.032
Oddy, W. H. (2002). The impact of breastmilk on infant and child health. Breastfeeding Review, 10(3), 5–18.
Ondari, E., Calvino-Sanles, E., First, N. J., & Gestal, M. C. (2021). Eosinophils and bacteria, the beginning of a story. International Journal of Molecular Sciences, 22(15), 8004. https://doi.org/10.3390/ijms22158004
Perdigon, G., De Moreno de LeBlanc, A., Valdez, J., & Rachid, M. (2002). Role of yoghurt in the prevention of colon cancer. European Journal of Clinical Nutrition, 56(3), S65–S68. https://doi.org/10.1038/sj.ejcn.1601490
Peterson, L. W., & Artis, D. (2014). Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. NATURE REVIEWS IMMUNOLOGY, 14(3), 141–153. https://doi.org/10.1038/nri3608
Riecken, E. O., Stallmach, A., Zeitz, M., Schulzke, J. D., Menge, H., & Gregor, M. (1989). Growth and transformation of the small intestinal mucosa—Importance of connective tissue, gut associated lymphoid tissue and gastrointestinal regulatory peptides. Gut, 30(11), 1630–1640. https://doi.org/10.1136/gut.30.11.1630
Ruffner, M. A., Ruymann, K., Barni, S., Cianferoni, A., Brown-Whitehorn, T., & Spergel, J. M. (2013). Food Protein-induced Enterocolitis Syndrome: Insights from Review of a Large Referral Population. Journal of Allergy and Clinical Immunology-in Practice, 1(4), 343–349. https://doi.org/10.1016/j.jaip.2013.05.011
Sen, P., Molinero-Perez, A., O’Riordan, K. J., McCafferty, C. P., O’Halloran, K. D., & Cryan, J. F. (2021). Microbiota and sleep: Awakening the gut feeling. Trends in Molecular Medicine, 27(10), 935–945. https://doi.org/10.1016/j.molmed.2021.07.004
Shek, L. P. C., Bardina, L., Castro, R., Sampson, H. A., & Beyer, K. (2005). Humoral and cellular responses to cow milk proteins in patients with milk‐induced IgE‐mediated and non‐IgE‐mediated disorders. Allergy, 60(7), 912–919. https://doi.org/10.1111/j.1398-9995.2005.00705.x
Sicherer, S. H. (2005). Food protein-induced enterocolitis syndrome: Case presentations and management lessons. Journal of Allergy and Clinical Immunology, 149–156. https://doi.org/10.1016/j.jaci.2004.09.033
Siegrist, C.-A. (2008). Vaccine immunology. In Vaccines (5th ed., pp. 17–36). Elsevier Philadelphia, PA.
Singh, G., Brass, A., Knight, C. G., & Cruickshank, S. M. (2019). Gut eosinophils and their impact on the mucus‐resident microbiota. Immunology, 158(3), 194–205. https://doi.org/10.1111/imm.13110
Spencer, S. P., Silva, E. G. L., Caffery, E. B., Carter, M. M., Culver, R. N., Wang, M., … Sonnenburg, J. L. (2022). Fermented foods restructure gut microbiota and promote immune regulation via microbial metabolites. bioRxiv, 2022.05. 11.490523. https://doi.org/10.1101/2022.05.11.490523
Su, K.-W., Cetinbas, M., Martin, V. M., Virkud, Y. V., Seay, H., Ndahayo, R., … Kramer, E. (2023). Early infancy dysbiosis in food protein‐induced enterocolitis syndrome: A prospective cohort study. Allergy. https://doi.org/10.1111/all.15644
Su, K.-W., Patil, S. U., Stockbridge, J. L., Martin, V. M., Virkud, Y. V., Huang, J.-L., … Yuan, Q. (2020). Food aversion and poor weight gain in food protein–induced enterocolitis syndrome: A retrospective study. Journal of Allergy and Clinical Immunology, 145(5), 1430-1437.e11. https://doi.org/10.1016/j.jaci.2020.01.001
Talari, K., & Goyal, M. (2020). Retrospective studies–utility and caveats. Journal of the Royal College of Physicians of Edinburgh, 50(4), 398–402.
Tamang, J. P., Cotter, P. D., Endo, A., Han, N. S., Kort, R., Liu, S. Q., … Hutkins, R. (2020). Fermented foods in a global age: East meets West. Comprehensive Reviews in Food Science and Food Safety, 19(1), 184–217. https://doi.org/10.1111/1541-4337.12520
Taştan, G. S., & Arslan, Z. (2023). Development of Acute FPIES Following FPIAP in a Breastfed Infant. https://doi.org/10.1177/00099228221113710
Toscano, M., De Grandi, R., Miniello, V. L., Mattina, R., & Drago, L. (2017). Ability of Lactobacillus kefiri LKF01 ( DSM32079) to colonize the intestinal environment and modify the gut microbiota composition of healthy individuals. DIGESTIVE AND LIVER DISEASE, 49(3), 261–267. https://doi.org/10.1016/j.dld.2016.11.011
Toygar, F., & Bakirhan, H. (2023). Can integrative, complementary alternative medicine, and integrative and functional nutrition practices have a place in nutrition management? DAHUDER Medical Journal, 3(4), 105–116. https://doi.org/10.56016/dahudermj.1353461
Vansickle, G., Powell, G., McDonald, P., & Goldblum, R. (1985). Milk-induced and soy protein-induced enterocolitis—Evidence for lymphocyte sensitization to specific food proteins. Gastroenterology, 88(6), 1915–1921. https://doi.org/10.1016/0016-5085(85)90019-8
Vieira Borba, V., Sharif, K., & Shoenfeld, Y. (2018). Breastfeeding and autoimmunity: Programing health from the beginning. American Journal of Reproductive Immunology, 79(1), e12778. https://doi.org/10.1111/aji.12778
Vinderola, G., Perdigón, G., Duarte, J., Farnworth, E., & Matar, C. (2006). Effects of the oral administration of the products derived from milk fermentation by kefir microflora on immune stimulation. Journal of Dairy Research, 73(4), 472–479. https://doi.org/10.1017/S002202990600197X
Walker, W. A., & Iyengar, R. S. (2015). Breast milk, microbiota, and intestinal immune homeostasis. Pediatric Research, 77(1), 220–228. https://doi.org/10.1038/pr.2014.160
Wang, L.-J., Yang, C.-Y., Chou, W.-J., Lee, M.-J., Chou, M.-C., Kuo, H.-C., … Li, S.-C. (2020). Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. EUROPEAN CHILD & ADOLESCENT PSYCHIATRY, 29(3), 287–297. https://doi.org/10.1007/s00787-019-01352-2
Wieërs, G., Belkhir, L., Enaud, R., Leclercq, S., Philippart de Foy, J.-M., Dequenne, I., … Cani, P. D. (2020). How probiotics affect the microbiota. Frontiers in Cellular and Infection Microbiology, 9, 454. https://doi.org/10.3389/fcimb.2019.00454
Woodruff, S. A., Masterson, J. C., Fillon, S., Robinson, Z. D., & Furuta, G. T. (2011). Role of eosinophils in inflammatory bowel and gastrointestinal diseases. Journal of Pediatric Gastroenterology and Nutrition, 52(6), 650–661. https://doi.org/10.1097/MPG.0b013e3182128512
Xing, L., Fu, L., Cao, S., Yin, Y., Wei, L., & Zhang, W. (2022). The Anti-Inflammatory Effect of Bovine Bone-Gelatin-Derived Peptides in LPS-Induced RAW264.7 Macrophages Cells and Dextran Sulfate Sodium-Induced C57BL/6 Mice. Nutrients, 14(7), 1479. https://doi.org/10.3390/nu14071479
Yilmaz, E. A., Soyer, O., Cavkaytar, O., Karaatmaca, B., Buyuktiryaki, B., Sahiner, U. M., … Sackesen, C. (2017). Characteristics of children with food protein-induced enterocolitis and allergic proctocolitis. Allergy Asthma Proc, 38(1), 54–62. https://doi.org/10.2500/aap.2017.38.4023
Yu, Y., Dunaway, S., Champer, J., Kim, J., & Alikhan, A. (2020). Changing our microbiome: Probiotics in dermatology. British Journal of Dermatology, 182(1), 39–46. https://doi.org/10.1111/bjd.18088
Zinöcker, M. K., & Lindseth, I. A. (2018). The Western diet–microbiome-host interaction and its role in metabolic disease. Nutrients, 10(3), 365. https://doi.org/10.3390/nu10030365
Appendix
Case 1 – FPIES
This boy was born via C-section in 2017. His mother followed the Weston A. Price diet (similar to the GAPS Diet, but soaked and fermented whole grains and legumes are not removed) for a few years prior to conception, and continued with this diet during pregnancy and lactation.
In the first 16 weeks of life the infant had eczema and terrible colic. Testing for allergies at five months of age found none. At eight months of age, solid foods were introduced but caused digestive discomfort. FPIES was diagnosed after reaction to introducing avocado. The infant developed severe vomiting, became pale and very weak within three hours after ingestion. Diarrhoea followed for two days with liquid and very pale looking stools. He had poor sleep.
At the age of 13 months, he received his first vaccinations against measles, mumps and rubella. Two weeks later, he had a severe reaction to sesame seed and was hospitalised. From then on blood tests revealed many different IgE allergies. The toddler received all vaccines required in the USA up to three years of age, each time experiencing high fever, rashes, and general malaise. Tests for allergies to vaccine components were performed showing reactions to all components; some of them triggered very severe reactions.
His growth rate was declining. The little boy was born at an average weight (60% weight curve), but his weight kept decreasing to reach the 3% weight curve at 32 months old. At this age, he had been diagnosed with 14 immunoglobulin E (IgE) mediated food allergies, FPIES to avocado labelled as fatal, and multiple environmental allergies. Blood tests showed high level of glyphosate and other chemicals in his body.
Since the introduction of solids this child was on the Weston A. Price diet; he has never eaten any processed food or followed the standard American diet, and he had always consumed high-quality food (organic produce and animal products from pasture-raised cattle).
At 32 months of age, he began following the GAPS Nutritional Protocol and started gaining weight. In three months, he reached the 20% weight curve and kept gaining weight to reach the 45% weight curve within six months from starting the GAPS Diet. After one week on stage one of The GAPS Introduction Diet, he moved through the rest of the stages. Rigorously following the GAPS Diet (Campbell-McBride 2010), the parents gradually added occasional fruits, honey, raw and fermented vegetables. Nuts were only added after having passed the specific oral food challenges.
In addition to a change of diet, the little boy has taken an antifungal medicine for 14 days (Diflucan) and natural supplements for chelation of toxic metals, removal of yeast and liver support. He was breastfed until four years of age and never had any cow-milk products.
At four years and nine months of age, the FPIES food challenge was conducted at the food allergy paediatric unit at Mt. Sinai hospital (New York, USA) under medical supervision, with an intravenous drip in place and all lifesaving drugs ready to be administered. The ingestion of half an avocado did not cause any reaction; the young boy therefore passed the test (avocado used to cause allergy, classed as fatal). This experience was very stressful for the family, but brought a great relief. Since then, the little boy started eating avocado regularly without any symptoms.
This child is now six years old and continues to follow the GAPS Nutritional Protocol. Recent allergy testing revealed normal total IgE levels for the first time in his life. He has overcome FPIES as well as IgE mediated allergies to eggs, almonds, macadamia nuts, hazelnuts, fish, shellfish, eggs and dairy products.
He now consumes home-made meat stock daily as well as plenty of animal fats, especially butter and cream. He consumes GAPS raw milk kefir daily and gets exposed to the sun between May and October. As advised by Dr. Campbell McBride (2010), yoghurt, cream and milk kefir are fermented for a minimum of 24 hours, as this ensures that lactose has been digested by the beneficial bacteria and casein has been broken down. This little boy lives in a chemical-free environment and all cleaning and hygiene products used in his house are natural. He only drinks filtered water and has a detoxifying bath every evening, either with Epsom salt, baking soda, magnesium flakes, seaweed, clay, Dead Sea salt, or apple cider vinegar.
His mother is very proud of her son’s growth and development, and she is happy to see him much healthier now.
Case 2 – FPIES
The mother of this girl changed her standard American diet for the Wise Traditions Diet (removing industrial food and refined sugar) six months prior to conception, and kept following it during pregnancy. Mother and infant took no form of medication during pregnancy, birth or thereafter, and the little girl was never vaccinated. The baby was born naturally at home at full term in 2013. She was breastfed and was developing normally. While exclusively breastfed, she was a happy baby, fed well and slept well. Around six months of age solid foods were introduced starting with avocado, egg yolk, grated liver and mashed banana. The infant vomited after the introduction of egg yolks. She then started reacting to other foods (chicken, beef, homemade yoghurt, etc.) with the worst reaction being to eggs. Each crisis would cause vomiting, diarrhea, paleness, and lethargy.
The parents started keeping a diary and realized that vomiting started two hours exactly after she would eat any trigger food. Suspecting FPIES, based on information found on the internet, the parents contacted a holistic doctor who diagnosed their daughter with FPIES. After one month of exclusive breast-feeding, the mother and infant started the GAPS Nutritional Protocol. At the time the infant was seven months old. She started drinking beef or chicken stock, one spoonful at a time. Following stage one of The GAPS introduction Diet, the child would eat only boiled zucchini, beef or chicken stock (two cups per day) and eventually tiny pieces of chicken or beef. More vegetables were gradually added to the diet. She would get bright red cheeks and her skin would flare up, but the parents felt that the protocol was helping. After a few months on stage one, their daughter moved through the rest of The GAPS Introduction Diet. They celebrated her first birthday with a meatball cupcake with a whipped cauliflower mash frosting, and the infant loved it. According to the child’s mother, progress was slow and gradual, but was leading to success.
Around one year of age, the little girl tried one drop of egg and vomited. This is when her mother tested her breastmilk for glyphosate, but found no detectable levels. At 15 months of age, the infant showed less interest in mother’s breastmilk, thus homemade raw fermented dairy products were gradually introduced: yoghurt and milk kefir fermented for at least 24 hours, occasionally butter and cheese made out of grass-fed raw A2 milk. By the age of 18 months, she could tolerate eggs without any reactions. Her parents started incorporating tiny drops of eggs into her meals. By the age of two years the little girl was on the Full GAPS Diet and stayed on it until three years of age.
She is now 10 years old. According to her mother, “she healed quickly and beautifully and has really been in vibrant health ever since three years of age”. Later on, she moved to the Wise Traditions Diet (Weston A. Price diet) with introduction of grains. She eats organic, homemade, fermented sourdough, grass fed meats and butter, in addition to raw dairy products. She tolerates all foods, eats out in a restaurant a few times a year and occasionally enjoys organic ice cream as well as an organic boxed mac & cheese product.
Her mother is very proud and happy with the success of the GAPS Nutritional Protocol in her family: “(My daughter) is so smart, working grade levels ahead in school. She has no skin issues and recovers well and quickly from sickness. She has a big round face and a huge bubbly personality. I’m so thankful for GAPS and the healing it brought us. I went on to have three more kids and they have not had any issues with foods.“
Case 3 – FPIES
This boy, born in 2020, has been exclusively breastfed since birth and has never had any cow-milk formula. He was an unhappy baby with reflux, bad sleep, eczema and various skin rashes. The mother started eliminating foods from her diet in order to decrease her son’s symptoms. She stopped eating dairy products, starchy foods, grains and poultry. Solid foods were introduced at the age of five months and a half, starting with potatoes, fruit and vegetables. At eight months of age the infant started reacting to mango, sweet potato and banana, finishing up in the emergency room with vomiting and lethargy. Worried, his parents ceased introducing new foods and started monitoring his reactions. Meanwhile, the mother had discovered the GAPS Nutritional Protocol and at nine months of age, her son started the GAPS Introduction Diet. His stools became more solid and eczema decreased. The little boy “started thriving” according to his mother. He was consuming homemade lamb meat stock, followed by introduction of vegetables, fish, lamb, and pork meat.
After his first birthday, he was given a teaspoon of chicken meat a few days in a row. This gave him his worst reaction ever: in two hours after ingestion, he had prolonged vomiting and become lethargic. This is when his parents contacted a Certified GAPS practitioner and asked for guidance in the GAPS Nutritional Protocol. The little boy started the No-Plant GAPS Diet, and his eczema and skin rashes disappeared. He was one year and a half old at the time. After a while on the No-Plant GAPS Diet, the infant was moved back to the first stage of the GAPS Introduction Diet. He soon went to stage two with the introduction of egg yolks, yoghurt, kefir and sour cream (fermented for longer than 24 hours). Dairy products were well tolerated, and the infant loved them. The family had to deal with a problem of excess moisture and mould in the house. To help his body detoxify, the boy had enemas and magnesium chloride baths. He also had a few supplements to support his liver.
Since then, he has been on the extended stage two of the GAPS Introduction Diet. He loves fish and fermented vegetables. The parents are planning to give the second stage of the GAPS Introduction Diet a few more months before going through another food challenge or FPIES testing. According to his mother, her son is growing and developing well, speaks well, has a very good memory, and is much healthier now.
Case 6 – FPIES
This girl was born naturally in 2012, was breastfed until three years of age and had never been fed any cow-milk formula. Her mother took antibiotics during pregnancy because she was not putting weight on.
The infant showed her first reaction at two months old, after having been vaccinated for diphtheria, tetanus, polio, Haemophilus influenzae type B, and pneumococcal disease. A trickle of blood appeared in her stools the following day. At four months of age, the infant was vaccinated again for the same diseases. This time the reaction was severe: she filled her diaper with blood and mucus and developed breathing difficulties six hours after the injection. The infant became consistently pale and developed a sleeping disorder. She stopped putting weight on and soon started losing weight.
Her parents were used to dealing with food allergies, as their older child was intolerant to lamb meat and soy. The mother, who exclusively breastfed her daughter, started eliminating foods from her own diet, but she could barely eat anything as the infant was still reacting to everything apart from lamb meat and quinoa. Testing for allergies at six months of age showed negative results, but FPIES diagnosis followed and doctor recommended to limit mother’s diet to lamb and quinoa. The parents saw less blood in her stools, but the little girl was now anaemic and was not putting weight on or growing. The mother tried to introduce solid foods (gluten-free grains, mashed vegetables and meat), but bleeding in the stool re-started. Antibiotics were given to the baby for an ear infection to which she reacted badly.
When the infant was seven months old, the parent heard about the GAPS Nutritional Protocol and decided to try it. They added meat stock to their daughter’s diet but this caused abundant blood in her stools. Trying meat stocks made with different meats, they finally found the right one (chicken and duck). The child started consuming meat stock, and parents started adding a drop of sauerkraut juice to the stock, gradually increasing to half a teaspoon per day. Bleeding through the stool ceased and the little girl started putting weight on. After several months, the infant could drink a cup of meat stock a day. The parents started adding some vegetables following the GAPS Introduction Diet strictly. The little girl first tolerated chicken and duck stock, then meat with a lot of duck fat, then peeled, seeded and boiled zucchini (the first vegetable she could tolerate), then pumpkins, onions and garlic. Skin reactions appeared with the introduction of onions, but these lasted for a very short time. According to the mother, reactions had been much more manageable since the implementation of the GAPS Protocol, with occasional skin reactions but far less digestive trouble. The child also had daily detoxifying baths.
Indeed, before starting the GAPS Diet, this baby had always had diarrhoea. In the early stages of the GAPS Nutritional Protocol, occasional constipation was managed with daily enemas. As the diet progressed, the stools normalised.
By a few months after her second birthday, the infant was on the Full GAPS Diet. Allergy testing revealed normal results. The little girl followed the Full GAPS Diet strictly until three and a half years of age. She is now 11 years old, follows the GAPS Diet most of the time, but she can occasionally eat fermented rice, fermented quinoa and sourdough bread. Occasionally she can tolerate other foods, when away on holiday for a week.
The girl’s mother wrote: “It was a very difficult process, but definitely life – changing experience; GAPS saved my baby’s life! The GAPS Protocol also solved health problems beyond expectations, such as ear infections, ENT problems and eczema. It improved the quality of life of our whole family. Nowadays my daughter is growing well, she is tall, strong and does well in school”.
Case 7 – FPIES
This girl, born in 2009, was breastfed until six months of age and never had any cow-milk formula. She received all her vaccinations at birth and a few months later. At two to three months of age, following vaccinations, her mother noticed a drastic change in her health and development. The infant seemed not to absorb nutrients from breast milk and stopped growing. At five months of age she had her first FPIES reaction triggered by the introduction of rice. The paediatrician diagnosed it as a virus and parents were advised to stop introducing solid foods for a month. At six months of age, upon introducing oats, the infant had her second FPIES episode: vomiting, diarrhea, eczema, paleness and stools rich in mucous. The infant was losing weight. This resulted in the diagnosis of FPIES and “failure to thrive”.
Doctors recommended to stop breastfeeding and instead use a medical formula as the bulk of her nutrition. This caused new symptoms, including behavioural issues, hyperactivity and poor impulse control. According to her mother, FPIES was “a result of vaccine injury”, although she feels that she had no choice but to follow the medical advice about vaccinations.
From six months to four years of age, this little girl was mostly fed with the medical formula. Foods were introduced by trial, one at a time, for one week each, until they were determined as safe. At one year of age, her only safe foods were cooked pears. Slowly and over time, her diet grew to include cooked and raw fruits, corn, peas, beans, wheat, vegan cheese, meat, and nuts. All dairy, eggs, rice and oats were avoided.
At four years old, the girl went through a food challenge at the hospital, in which she nearly died. A mixture of 2% milk flavoured with artificial and sweetened powder was being introduced, which she reacted to with a full-blown FPIES emergency. Once the child was stabilized, her mother knew a change was required.
Her mother explains: “I then discovered that the medical formula (my daughter) had been on from infancy contained aspartame, which led me to seeking an alternative”. Online research led her to a blog of a mother who healed her son’s FPIES with the GAPS Nutritional Protocol. She began implementing it right away, starting with the GAPS Introduction Diet. At that time, her daughter was four and a half years old. The switch from formula to homemade meat stock changed her life radically. All of her behavioural issues went away “almost overnight”, according to her mother. Within two months of the GAPS Introduction Diet, the little girl was able to eat two of her known trigger foods: dairy and eggs. She stayed on the second stage of the GAPS Introduction Diet for a few months before slowly introducing other foods, that were FPIES and IgE triggers. The skin sensitivity test recommended in the GAPS Protocol, consisting of placing a few drops of the product in the inner part of the wrist overnight to test for any reaction (Campbell-McBride, 2020, p.145), was used for these foods before introducing them in tiny amounts; the girl showed no reactions. After three years on the GAPS Protocol, she was able to eat all of her other known food triggers (including rice and oats, prepared according to the Weston A. Price method).
As part of the GAPS Nutritional Protocol, iodine paint was used in addition to daily detoxifying baths (adding some Epsom salt, baking soda or apple cider vinegar to the bath).
The girl is now 14 years old. Her diet is still based on the Full GAPS Diet / Weston A. Price diet, but she tolerates all other foods. She can now go to restaurants on occasion or be invited by friends and family, where she can eat everything offered, even with conventional and processed ingredients.
Case 8 – FPIES
This girl, born in 2009, had severe colic from birth and by ten weeks of age had blood in her stool. Exclusively breastfed until 12 weeks of age, she was then diagnosed with milk/soy protein intolerance and was given an amino acid formula. Solids were tried at eight months of age, but the infant reacted badly to every food with profuse vomiting, diarrhoea, manic moods, fever or lethargy. She became pale, and had skin rashes and mucus in her stool. She had sleep issues, reflux, was an unhappy baby, had baby acne and suffered from hyperactivity, poor impulse control and hypotonia. She was tested for allergies and by the age of ten months was diagnosed with FPIES. This little girl was one of the first babies nationwide to receive this diagnosis. According to her mother, at that time the definition of the diagnosis was “allergic to food”.
Despite being on the elemental formula, symptoms worsened. Vomiting and constipation were very frequent and chronic respiratory problems made her wheeze. She had a distended belly and full-body eczema. She refused food. She was diagnosed with colitis and sugar intolerance. At that time, all blood and skin tests showed no allergy, but her skin was so sensitive that contact with diaper cream or body soap would cause vomiting, diarrhoea and shock-like symptoms, as well as swollen eyes and hives.
At 16 months old, the toddler was diagnosed with corn allergy. As her elemental formula contained corn, her parents were advised to wean the little girl off the formula and feed her with home-made almond milk and other foods. Almond milk caused uncontrollable vomiting and dehydration. The infant lost 22 ounces very quickly. This is when her mother remembered having heard of the GAPS Nutritional Protocol for FPIES. In her words, she finally found hope to heal her baby.
At 18 months of age, and after four weeks of weaning from her formula, the little girl started the GAPS Introduction Diet. According to her mother, “within 24 hours of her last formula bottle, she went from an autism evaluation referral to saying six new words. Within three months she had caught up on all milestones and regained all lost weight.” The infant stayed on stage one of the GAPS Introduction Diet for 18 months, during which time the only vegetable she could eat was zucchini. The introduction of raw and fermented coconut water was “pivotal” according to her mother: “once she got (raw and) fermented coconut water, she began to have body biome change, even more so than (with) the oral antifungal medication”. After these 18 months on stage one of the GAPS Introduction Diet, the little girl could slowly move through other stages. “Now she dances, she runs, she thrives”, says her mother in a video, which she made about their healing journey. “We no longer make regular trips to the doctor’s office for oxygen level checks, we no longer fear for the vomit, or pain, or crying that comes after trying a new food, we no longer panic over cross-contamination in our home kitchen.”
At three years of age, the little girl could tolerate many more vegetables and had not shown any FPIES symptom for more than a year. At the age of three years and a half, she started tolerating some dairy products (goat milk ghee), after about two years on the GAPS Nutritional Protocol. At about four years old, she stopped having FPIES symptoms to new GAPS foods introduced. This young girl is now 14 years old and she lives in a small farm in Northern California. She is a healthy teenager, as long as she follows the Full GAPS Diet, allowing occasional treats. She goes through the GAPS Introduction Diet again periodically. She still has FPIES reactions when she stays off the GAPS Nutritional Protocol for too long, especially when she consumes soy. Her mother has been so convinced with the effectiveness of the GAPS Nutritional Protocol for healing FPIES, that she became a Certified GAPS Practitioner. She created an online community to help other parents dealing with FPIES. She concludes, about her daughter’s journey: “She could eat nothing. Eating ANY food gave her an FPIES reaction, and being able to eat ANY food meant that she had overcome an FPIES reaction and was healing. GAPS saved my daughter’s life”.


