JOM Archives – Volume 22, Number 3, 2007
Introduction
For many years, The Center for the Improvement of Human Functioning has used the infusions of 15-50 g of vitamin C (ascorbic acid, ascorbate, AA) intravenously as an adjunctive therapy for cancer patients. The first experiments studied the effect of vitamin C on cultured tumor cells and tumors in animal tests and found that AA is preferentially toxic to tumor cells. (Riordan, Hunninghake, Riordan, et al., 2003; Casciari, Riordan, Schmidt, et al., 2001; Riordan, Riordan & Casciari, 2000; Riordan, Jackson & Riordan, 1996; Jackson, Riordan, Hunninghake, et al., 1995) Results of these studies showed that high doses of AA are useful in the treatment of cancer patients. We analyzed the pharmacokinetics of vitamin C after 15-50 g of intravenous infusion and developed an infusion protocol. (Riordan, Hunninghake, Riordan, et al., 2003)
Over the past years, the health benefit of high doses of AA has been the subject of debate.
Many studies have demonstrated that ascorbic acid is the most effective water- soluble antioxidant in human plasma. (Frei, England & Ames, 1989; Frei, Stocker & Ames, 1988; Frei, Forte, Ames, et al., 1991; Padayatty, Levine, 2001) A number of papers raised concerns that high doses of AA induce pro-oxidant effect, cause genetic damage of cells, (Podmore, Griffiths, Herbert, et al., 1998; Cai, Koropatnick & Cherian, 2001; Guadarelli, De Sanctis, Cellini, et al., 2001) and increase oxidative stress in plasma and cell environment.
For low doses, the pro-oxidant and anti-oxidant effects of AA have been a subject of many investigations. Several studies indicated that in the presence of redox–active transition metals, AA could act as a pro-oxidant. (Aisen, Cohen & Kang, 1990; Borg, Schaich, 1989) This phenomenon is based on the ability of ascorbate metal-dependent production of hydroxyl and alkoxyl radicals in vitro by Fenton chemistry. (1) In vitro, the combination of ascorbic acid, hydrogen peroxide, and transition metals forms a highly pro-oxidant mixture generating hydroxyl radicals.
In addition, the removal of an electron from AA results in the generation of ascorbic acid free radical. Removal of another electron by reactive oxygen species (ROS) or ascorbic free radical results in the formation of dehydroascorbic acid. Dehydroascorbic acid is either reduced to ascorbic acid or hydrolyzed to di-ketogulonic acid. (Bianchi, Rose, 1986; Rose, Choi & Bode, 1992) Ascorbic free radical is a relatively stable radical; however, it can cause the oxidative damage of cellular components, including those associated with lipid peroxidation.
The pro-oxidant function of vitamin C was demonstrated in reactions when AA reduced transition metal ions like Fe3+ and Cu2+ which, in the presence of hydrogen peroxide, generate the hydroxyl radical.
The other studies in human plasma demonstrated that even in the presence of redox–active iron or copper and hydrogen peroxide, AA acts as an antioxidant that prevents lipid peroxidation and does not promote oxidation in human plasma. (Suh, Zhu & Frei, 2003) The measurements were performed in physiological concentrations of AA. The prevention of lipid peroxidation was proven by measuring protein carbonyl formation and the depletion of reduced thiols.
There are many different antioxidant components in plasma. The most important biological antioxidants would appear to be AA, vitamin E, uric acid, glutathione peroxidase, catalase, superoxide dismutase, transferrin, and ceruloplasmin. The goal of this research was to analyze the effect of high doses of AA supplementation on the antioxidant capacity of plasma and the resistance of the plasma to oxidative stress. This experiment measured the total level of antioxidant capability of plasma instead of measuring the antioxidant effect of AA only. This is because the synergistic effect of antioxidants and evaluation of the level of each antioxidant does not show the total antioxidant status. The synergistic effect of antioxidants can be demonstrated by the following example. If the stoichiometric factor of ascorbate is 0.7 and ascorbate recycles tocopherol, which has a stoichiometric factor of 2, then the effect of ascorbate supplementation on the antioxidant status of plasma will be higher than a simple comparison of the levels of these antioxidants.
In our study, we analyzed the total peroxyl-radical trapping capacity of plasma after treatment of patients with high doses of AA and in vitro experiments by addition of AA in plasma in doses that are achieved after 15-50 g of IV AA.
Methods
Measurements of the Antioxidant Capacity of Plasma
The total level of antioxidant capacity of plasma was determined by fluorescence method TRAP (The Total Radical-Trap- ping Antioxidant Parameter). (Delange, Glazer, 1989; Ghiselli, Serafini, Maiani, et al., 1995) TRAP measures the total peroxyl radical–trapping capacity, and practically all chain- breaking antioxidants are included in these measurements. The procedure is based on the properties of reagent 2,2’-diazobis (2-amidinopropane) dihydrochloride (ABAP). It produces peroxyl radicals at a constant rate. The peroxyl radicals produced in this way have sufficient energy to remove hydrogen from a lipid substrate, thus inducing a lipid peroxidation chain reaction, and they are able to oxidize fluorescent protein R-phycoerythrin (R-PE).
The addition of plasma antioxidants in the reaction mixture protects proteins R-PE from oxidation. The lag phase induced by plasma was compared with 6-hydroxy-2,5,7,8, tetramethy-lchroman- 2-carboxylic acid (Trolox, a water-soluble analogue of vitamin E).
All reagents and the reaction mixture were prepared in buffered saline without ions by treating the buffer with Chelex 100 (Bio-Rad Laboratories). After mixing the buffer and Chelex 100 for about one hour and filtration, the buffer was stored in plastic tubes no longer than 2 weeks. Degradation of R-PE was measured every 10 minutes and cuvettes were kept at a constant temperature of 37°C.
The reaction mixture consisted of 1.5 10-8 M R-PE diluted in 2 mL 75 mM phosphate buffer, pH=7.0 without ions. Eight μL of plasma or 30 μL of 120 μM Trolox were added to 2.0 mL final volume. The solution was maintained at 37°C for five minutes in 10 mm quartz fluorometer cells. The oxidation reaction was started by adding ABAP 0.03 g per 2 mL of buffer without ions.
The level of emission was measured by spectrofluorometer Fluorolog-3 (SPEX Company). The excitation wavelength was 540 nm and the emission of protein R-PE was measured in the range of 560 nm -590 nm with the maximum emission at 573 nm. The measurements demonstrated a reproducible decrease in the emission of protein over time due to oxidation.
Measurements of the Contribution of Ascorbic Acid in Plasma Antioxidant Capacity by the Use of Ascorbate Oxidase
To estimate the contribution of ascorbate to the plasma antioxidant capacity, plasma was treated by ascorbate oxidase. (Delange, Glazer, 1989) Briefly, 100 μL of plasma were added to 200 μL of 50 mM phosphate buffer at pH=5 and incubated with 0.5 U/mL of ascorbate oxidase for 30 minutes at 25 °C. At the end of the incubation, a neutral pH was restored. Measurements were performed for treated and untreated plasma. Dilution of plasma was the same in all samples (1:250).
Sample Preparation
Human plasma was obtained from healthy volunteers and patients and collected in BD vacutainer EDTA tubes. The project was reviewed and approved by The Center’s IRB committee. EDTA treated tubes were immediately centrifuged at 3000 g for 15 minutes, kept on ice and assayed for TRAP. For comparison of emission curves with and without proteins, proteins were removed from plasma by precipitating them by ammonium sulphate. In most experiments, plasma was measured with proteins, as we found that removal of proteins changed the antioxidant capacity of plasma, probably as the result that lipid soluble antioxidants, such as vitamin E, precipitated along with proteins.
Results
In Vitro Effect of Ascorbic Acid Supplementation on the Plasma Antioxidant Capacity
According to TRAP procedure, the addition of plasma in the reaction mixture protects protein R-PE from oxidation. The time of protection depends on the anti- oxidant capacity of plasma. Incubation of human plasma at 37°C with the water- soluble initiator ABAP, led to depletion of the plasma antioxidants. The depletion of antioxidants of plasma due to oxidants can be seen at the slope of the emission curve (Figure 1). The time of protection, or lag phase, was calculated at the 0.9 level of fluorescent protein intensity from the initial level.
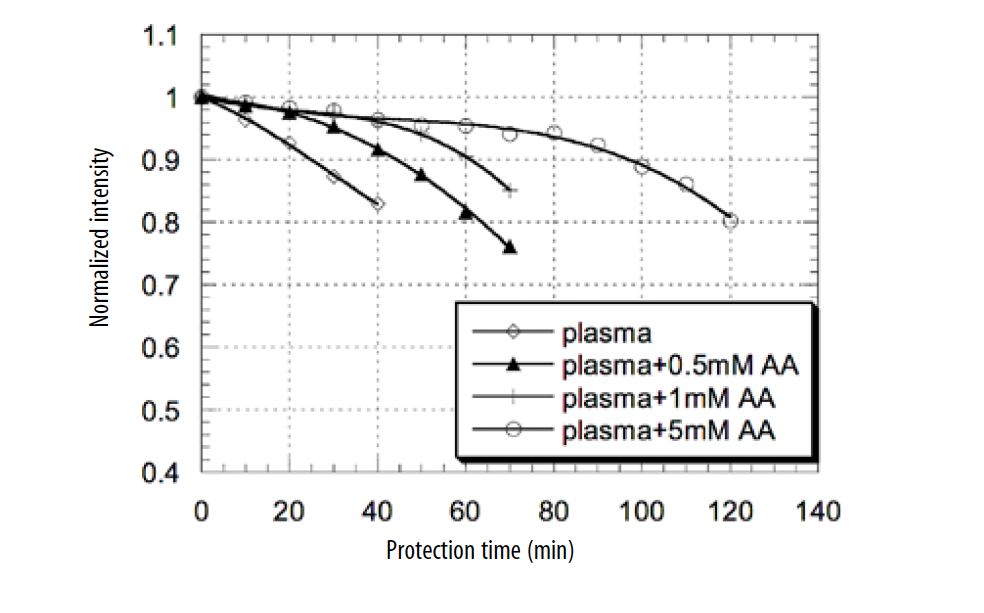
Figure 1. Antioxidant defenses of plasma supplemented by different concentrations of ascorbic acid exposed to water–soluble radical initiator ABAP.
In our experiments, different concentrations of AA were added to plasma. Time of R-PE protection was measured for concentrations of AA 50 μM, 100 μM, 200μM, 1mM, 3mM, and 5mM. The effect of the addition of different concentrations of AA to plasma on R-PE fluorescence decay is presented in Figure 1. The curves presented in Figure 1 demonstrate the fluorescent intensity of proteins decreases, over time, after oxidation in reaction mixture with radical initiator. The higher level of antioxidant leads to the longer time of protection of fluorescent protein from oxidation. The lowest level of protection to radical initiator ABAP was measured for plasma without addition of AA (25 minutes of protection for level 0.9 of normalized intensity). The addition of 0.5mM of AA to plasma increased time of fluorescent protein protection to 45 minutes (1.8 times). The addition of 1mM of AA to plasma increased the time of protection to 60 minutes (2.4 times) and the addition of 5 mM of plasma increased the time of protein protection to 100 min (4 times).
According to these data, supplementation of plasma in vitro with an increasing amount of AA led to an increase of the lag phase proceeding to R-PE oxidation and lipid peroxidation.
The time of protection against peroxyl radicals was normalized at the time of protection of standard (Trolox) and calculated as the difference between the time of protection of plasma with exogenous AA and protection time of plasma with endogenous AA. The calculated time of protection demonstrated that the addition of AA in plasma increased the antioxidant effect proportional to the added AA. The dependence of the time of protection on the concentration of exogenous ascorbic acid is presented in Figure 2.
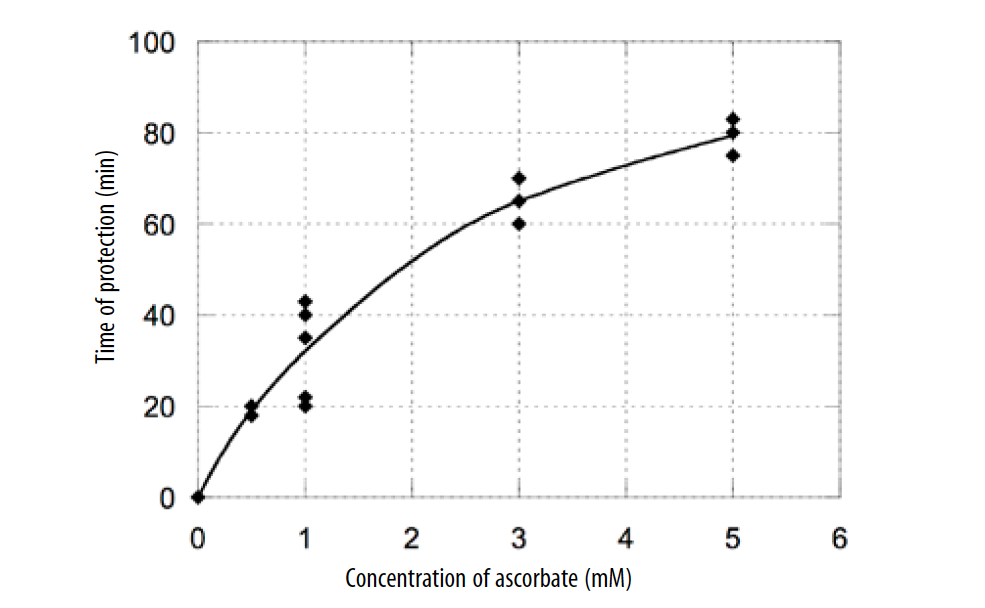
Figure 2. Dependence of the time of antioxidant defense on the exogenous concentration of ascorbic acid.
According to these data, the time of protection increased with increasing AA concentration in plasma and ascorbate had antioxidant activity for all measured values in the range of 50 μM-5 mM. This range includes the typical natural concentration of AA in plasma (about 50 μM) and the level of AA in plasma after 15 g of intravenous AA treatment.
As it can be seen from graph, there is non-linearity of the protection time with ascorbate concentration for high doses of AA. We explored the possible causes of the nonlinearity of protection time with ascorbate concentration. Experiments were repeated with ascorbate- supplemented buffer in place of ascorbate –supplemented plasma. According to our measurements, this effect was caused by the presence of trace metal ions in the buffer. The presence of trace metal ions in the buffer may cause the formation of free radicals from AA and additional oxidation of fluorescent protein R-PE. Another factor may be that the stoichiometric factor for AA depends on the initial concentration of AA and decreases with increased concentration. (Wayner, Burton, Ingold, et al., 1987)
Antioxidant Capacity of Plasma Before and After IVC
Intravenous doses of AA produce plasma concentrations much higher than the maximum tolerated oral doses. According to our previous study, infusion of 15 g of ascorbate during 45 minutes usually produces a maximum plasma concentration about 120 mg/dL (6.8 mM). Infusion of 60 g of ascorbate during 160 min may produce a maximum concentration in plasma near 300 mg/dL (17 mM). With in vitro experiments, we demonstrated that an ascorbate concentration of 140-400 mg/ dL induced a 50% decrease in the number of cancer cells (different cell lines had different resistance to ascorbic acid). (Mikirova, Jackson, Casciari, et al., 2001)
To evaluate whether intravenous ascorbate infusion, which may have anti- tumor activity, does not have pro-oxidant effects on plasma lipids and proteins, TRAP assay was applied to measure the antioxidant capacity of plasma for several patients before and after treatments by intravenous vitamin AA. The experimental approach used to measure antioxidant capacity of plasma before and after IV AA was as described before. The analysis was performed at the same experimental conditions for plasma, which was taken before and after IV AA. Eight μL of plasma were added in the reaction mixture with R-PE and ABAP, and the protection time was measured at 10 minute intervals.
An example of the plasma protection time before and after 15 g of IV AA is shown for a healthy subject and a patient with liver and lung cancer (Figure 3). In addition, the oxidation of R-PE proteins without addition of plasma is presented as a control.
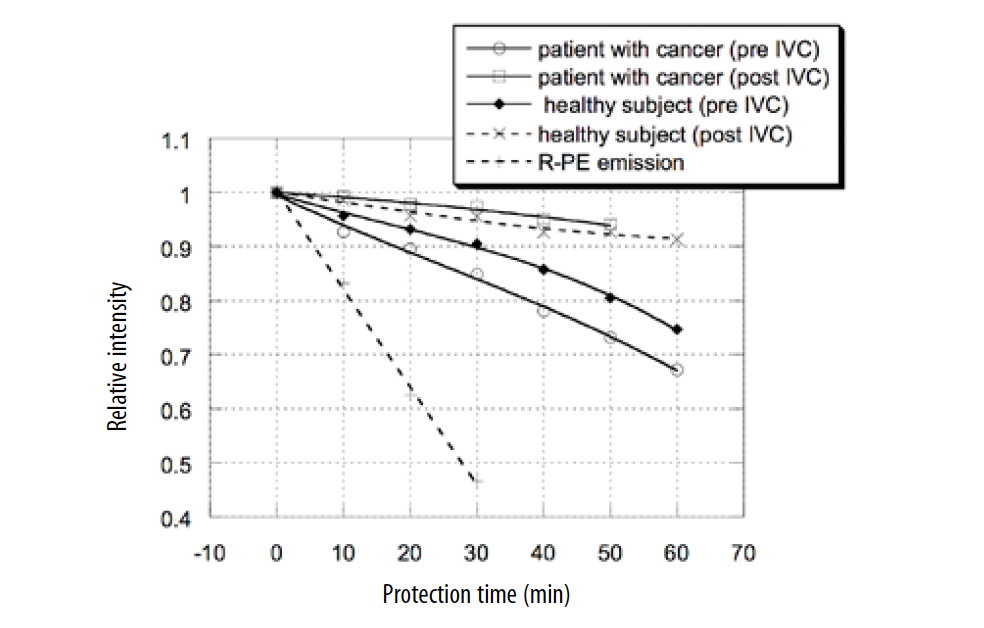
Figure 3. Effect of 15 g IV C on time of plasma protection against oxidative stress.
According to these data, the plasma level of antioxidant protection was 30% lower in the cancer patient before IV AA in comparison with the healthy subject, and it was improved after treatment. The peroxyl–radical trapping ability of plasma was increased 3 times after 15 g IV AA treatment of a cancer patient. For the healthy subject, the level of peroxyl trapping ability was improved 2 times, and the plasma response curves to oxidative stress were similar for the cancer patient and the healthy volunteer after IV AA treatment.
The lower level of antioxidant capability of plasma for the patient with cancer may be explained by the higher level of oxidative stress and the plasma level of lipid peroxidation products in patients with early and advanced cancer in comparison with healthy subjects. (Hristozov, Gadjeva, Vlaykova, et al., 2001; Kung, 2002) The increased level of oxidative stress increases the consumption of antioxidant molecules and enzymes.
The level of antioxidant protection after 15 g IV AA was measured for five patients and two healthy volunteers. Ac- cording to these measurements 15 g of IV AA improved the antioxidant properties of plasma two to four times. For all plasma samples, the level of AA was measured by HPLC with an electrochemical detector. After 15g of IVAA, the level of AA was in the range of 60-90 mg/dL (3.4 mM-5.1 mM).
The level of antioxidant protection of plasma depended on the dosage of IV AA. We measured serum from five patients treated with 15 g of IV AA, two patients treated with 25 g of IV AA, and two patients treated with 50 g of IVAA to plot the dependence of the antioxidant capacity of plasma on the dosage of IV AA injection (Figure 4)
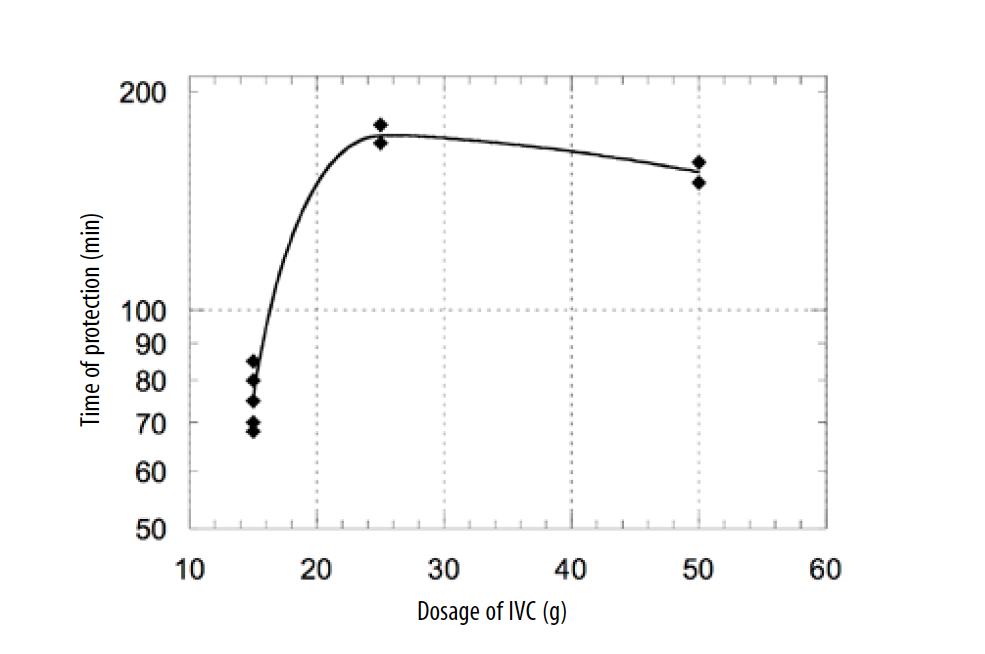
Figure 4. Dependence of the antioxidant capacity of plasma on the dosage of IVC.
According to these data, the treatment of patients intravenously with dosages of 15-25 g of AA resulted in the increased antioxidant capacity of plasma, and the level of antioxidant capacity correlated with the level of supplementation. For higher doses, the time of protection was not improved due to plasma saturation with AA.
To estimate the contribution of ascorbic acid in the total antioxidant capacity of plasma, AA was removed from serum by ascorbate oxidase. Treated and untreated plasma were added in the reaction mixture with the same dilution and the antioxidant capacity was measured by TRAP assay. The contribution of ascorbic acid in the total antioxidant capacity of plasma was in the range of 10-15%.
Conclusion
High-dose AA treatment is beneficial in a variety of clinical situations, when overproduction of reactive oxygen species is a critical pathological factor. Because free-radical damage and the formation of lipid peroxides are suspected in carcinogenesis and other pathological conditions, ascorbic acid supplementation may be useful for disease prevention.
According to these results, even high doses of ascorbate do not cause oxidative damage in plasma and can prevent the initiation of peroxidative damage to plasma lipids and proteins by aqueous peroxyl radicals. The level of protection time was improved by increasing the concentration of AA in plasma.
The peroxyl-trapping ability of plasma was increased 3 times after 15 g of IV AA treatments of a cancer patient. The level of antioxidant capacity correlated with the doses of AA in vitro experiments and in vivo after treatment of patients with 15-25 g of IVC.
Ascorbic acid can directly scavenge oxygen free radicals with and without enzyme catalysts and can indirectly scavenge them by recycling tocopherol to the reduced form. Reacting with activated oxygen more readily than other aqueous components, ascorbate protects critical macromolecules in cells and lipids and proteins in plasma from oxidative dam- age. According to our measurements, the contribution of ascorbic acid in total antioxidant capacity of plasma at the physiological level for endogenous AA is in the range of 10-15%.
As the result of our studies, we can conclude that AA is very important and is a primary antioxidant in plasma, protecting serum lipid peroxidation and protein oxidation. In addition, according to these results, high doses of AA increase the level of antioxidant capacity of plasma and do not have pro-oxidant effects on plasma lipids and proteins.
References
Aisen, P., Cohen, G. & Kang, J.O., (1990) Iron Toxicosis, International Review of Experimental Pathology, 31, 1-46
Bianchi, J., Rose, R.C., (1986) Dehydroascorbic Acid and Cell Membrane: Possible Disruptive Effects, Toxicology, 40, 75-82
Borg, D. C., Schaich, K. M., (1989) Pro-Oxidant Action of Antioxidants in CRC Handbook for Free Radicals and Antioxidants in Biomedicine, (Volume 1), Boca Raton, Florida: CRC Press
Cai, L., Koropatnick, J. & Cherian, M.G., (2001) Roles of Vitamin C in Radiation –Induced DNA-Damage in Presence and Absence of Copper. Chemico-Biological Interactions, 137(1), 75-88
Casciari, J.P., Riordan, N.H., Schmidt, T.L., et al., (2001) Cytotoxicity of Ascorbate, Lipoic Acid, and Other Antioxidants in Hollow Fiber In Vitro Tumors, British Journal of Cancer, 84(11), 1544-1550
Delange, R.J., Glazer, A.N., (1989) Phycoerythrin Fluorescence-Based Assay for Peroxyl Radicals: A Screen for Biologically Relevant Protective Agents, Analytical Biochemistry, 177(2), 300-306
Frei, B., England, L. & Ames, B., (1989) Ascorbate is An Outstanding Antioxidant in Human Plasma, Proceedings National Academy Sciences of the United States of America, 86(16), 6377- 6381
Frei, B., Forte, T., Ames, B., et al., (1991) Gas Phase Oxidants of Cigarette Smoke Induce Lipid Peroxidation and Changes in Lipoprotein Properties in Human Blood Plasma: Protective Effects of Ascorbic Acid, Biochemical Journal, 277(1), 133-138
Frei, B., Stocker, R. & Ames, B., (1988) Antioxidant Defenses and Lipid Peroxidation in Human Plasma, Proceedings National Academy Sciences of the United States of America, 85(24), 9748-9752
Ghiselli, A., Serafini, M., Maiani, G., et al., (1995) A Fluorescence-Based Method for Measuring Total Plasma Antioxidant Capability, Free Radical Biology and Medicine, 18(1), 29-36
Guadarelli, A., De Sanctis, R., Cellini, B., et al., (2001) Intracellular Ascorbic Acid Enhances the DNA Single-Strand Breakage and Toxicity Induced by Peroxynitrite in U937 Cells, Biochemical Journal, 365(2), 509-513
Hristozov, D., Gadjeva, V., Vlaykova, T., et al., (2001) Evaluation of Oxidative Stress in Patients with Cancer, Archives of Physiology and Biochemistry, 109(4) 331-336.
Jackson, J.A., Riordan, H.D., Hunninghake, R.E., et al., (1995) High Dose Intravenous Vitamin C and Long Time Survival of a Patient With Cancer of Head of the Pancreas, Journal of Orthomolecular Medicine, 10(2), 87-88
Kung, D.H., (2002) Oxidative Stress, DNA Damage and Breast Cancer, AACN Clinical Issues, 13(4), 540-9
Mikirova, N.A., Jackson, J.A., Casciari, J.J., et al., (2001) The Effect of Alternating Magnetic Field Exposure and Vitamin C on Cancer Cells, Journal of Orthomolecular Medicine, 16(30), 177-182
(16) Symposium: Prooxidant Effect of Antioxidant Vitamins, American Institute of Nutrition. J Nutr, 1996; 126: 1197S-1200S
Padayatty, S., Levine, M., (2001) New Insights Into Physiology and Pharmacology of Vitamin C, Canadian Medical Association Journal, 164(3), 353-355
Podmore, I.D., Griffiths, H.R., Herbert, K.E., et al., (1998) Vitamin C Exhibits Pro-Oxidant Properties, Nature, 392, 559
Riordan, H.D., Hunninghake, R.E., Riordan, N.H., et al., (2003) Intravenous Ascorbic Acid: Protocol for its Application and Use, Puerto Rico Health Sciences Journal, 22(3), 287-290
Riordan, N.H., Jackson, J.A. & Riordan, H.D. (1996) Intravenous Vitamin C in A Terminal Cancer Patient, Journal of Orthomolecular Medicine, 11(2), 80-82
Riordan, N.H., Riordan, H.D. & Casciari, J.P., (2000) Clinical and Experimental Experiences with Vitamin C, Journal of Orthomolecular Medicine, 15(4), 201-213
Riordan, N.H., Riordan, H.D., Meng, X.L., et al., (1995) Intravenous Ascorbate as a Tumor Cytotoxic Chemotherapeutic Agent, Medical Hypothesis, 44(3), 207-213
Rose, R.C., Choi, J.L., Bode, A.M., (1992) Short-Term Effects of Oxidized Ascorbic Acid on Bovine Corneal Endothelium and Human Placenta, Life Sciences, 50(20), 1543-1549
Suh, J., Zhu, B., Frei, B., (2003) Ascorbate Does Not Act as a Pro-Oxidant Towards Lipids and Proteins in Human Plasma Exposed to Redox-Active Transition Metal Ions and Hydrogen Peroxide, Free Radical Biology & Medicine, 34(10) 1306-1314.
Wayner, D.D.M., Burton, G.W., Ingold, K.U., et al., (1987) The Relative Contribution of Vitamin E, Urate, Ascorbate and Proteins to the Total Peroxyl Radical-Trapping Antioxidant Activity of Human Blood Plasma, Biochemica et Biophysica Acta, 924(3), 408-419


