Introduction
Chronic kidney disease (CKD) is usually antecedent to the most common cause of death, cardiovascular complications involving coronary artery disease, heart failure, and/or sudden cardiac death which are considerably higher in CKD than in the general population (Jankowski, 2021). The risk for cardiovascular complications escalates dramatically in stage 4 CKD (15-30 eGFR), where cardiovascular disease becomes the primary cause of mortality, surpassing end-stage renal disease (Rai, 2023). This happens because CKD induces a persistent systemic proinflammatory state, leading to the development of atherosclerotic lesions, vascular calcification, myocardial fibrosis, and vascular aging. The earlier CKD can be addressed with effective therapies, the longer an individual is likely to live.
Currently, there is no known cure for CKD, and medications only have limited effectiveness in slowing disease progression. Current treatments involving blood pressure control and glycemic control only delay progression to end-stage renal disease (ESRD) at best. Blood pressure medications are now known to potentially cause damage to the kidney (Watanabe, 2021). Finally diagnosis is commonly only made during the later stages of CKD when currently available treatments are ineffective while patients with ESRD are limited to dialysis or kidney transplantation (Chronic Kidney Disease, n.d.).
The current dogma is that CKD is like a permanent scar, whose progression can only be slowed, but that CKD can never be improved, and normal kidney function can never be restored. However, we have observed reversal of stage 3 CKD to stage 1 and stage 4 reversed to stage 2 when using a simple combination of biochemical agents based on basic biomedical science that effectively and directly address hyperphosphatemia, metabolic acidosis, and proteinuria. At this point, we have observed over 25 documented cases, and continuous basic and clinical research, as well as dozens of clinical trials, have provided substantial evidence that niacin with sodium bicarbonate directly addresses the needs of the typical chronic kidney disease patient (McConnell & Penberthy, 2021)
Study Rationale and Aim
When kidney function declines, the body’s ability to efficiently clear phosphorus becomes compromised and serum phosphorus rises, at which point it binds with calcium to initiate pathogenic processes. It is critical to reduce serum phosphorus levels, particularly in patients currently undergoing dialysis, since excessive phosphorus is a primary trigger for vascular calcification and is also harmful to nearly every organ system in the body as a whole.
Fortunately, there are straightforward and safe strategies, such as incorporating modest doses of immediate-release niacin (IR-Niacin), that may help reverse CKD in many patients (unpublished observations). A wealth of clinical research has already demonstrated that simple niacin or niacinamide treatment is remarkably effective in reducing hyperphosphatemia, in part due to the inhibition of the sodium phosphate transporter (Berns, 2008; McConnell & Penberthy, 2021; Nicotinic Acid and Nicotinamide: A Review of Their Use for Hyperphosphatemia in Dialysis Patients – Rennick – 2013 – Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy – Wiley Online Library, n.d.). Niacin turns out to be one of the most effective methods for addressing hyperphosphatemia, even at as little as 100 mg of niacin.
This niacin finding, however, is just the tip of the iceberg, as niacin’s potential benefits extend far beyond managing phosphate levels in the blood. Fibroblast Growth Factor-23 (FGF-23) is the only biomarker reflecting the pathology behind long-term exposure to elevated phosphorus and FGF-23 is known to be decreased by administration of niacin (Rao, 2014). Niacin is one of three forms of vitamin B3 by virtue of the fact that it serves as a biochemical precursor to the essential molecule nicotinamide adenine dinucleotide (NAD). NAD is essential to cellular life and is required in over 400 known gene functions in man. This includes essential roles in glycolysis and oxidative phosphorylation, which are the primary ways that cells make ATP for all energy. NAD is used in all of the phase 1 detoxification cytochrome P450 enzymes and too many more functions to mention. Many of these genes are required for human cellular life and we are still regularly discovering new therapeutic benefits when using niacin therapy to treat various indications in clinical medicine (Al-Amrani, 2024; Pirinen, 2020).
Niacin clinical studies consistently and continuously yield positive results (supplementary table 1). This is far more than any other vitamin-associated molecule. Even more, NAD levels are well known to be exceptionally low in many pathogenic disease processes (Braidy, 2013; Csiszar, 2019; Lautrup, 2024; Migliavacca, 2019; Poljšak, 2023; Song, 2024). This includes a review specifically focused on “NAD+ Metabolism Interventions in Premature Aging and Chronic Kidney Disease” that was published in 2022 (Chanvillard, 2022). The field of NAD biology continues to increase in recognition and appreciation at the basic science level. The stage is now set for the realization of NAD’s utility in the real-world clinical setting, and CKD is next.
Sodium bicarbonate (baking soda) is another safe and basic biochemical molecule that has proven in clinical trials to be effective in the treatment of CKD. A landmark study completed in 2009 demonstrated an 80% reduction in the transition to stage 5 / ESRD in patients with stage 3 or 4 CKD who were administered 1.8g of sodium bicarbonate (Baking Soda May Slow Progression of Chronic Kidney Disease, n.d.; de Brito-Ashurst, 2009). Optimal dosing of sodium bicarbonate involves BID administration with food, with 600mg taken at lunch and 1,200mg taken at dinner each day.
We have anecdotal and underlying evidence, and we are going to test it in the real world. The aim of this study is to assess the efficacy of the formulation for the treatment of stage 3 to 5 chronic kidney disease (eGFR 10-45 mL/min/1.73 m2) by monitoring both the estimated glomerular filtration rate (eGFR) and urine albumin-creatinine ratio (UACR), which provide an established measure of CKD stages. With this study, we will convert anecdotal evidence into scientific evidence.
We have formulated a single pill that combines these ingredients, together with isoquercetin, as a simplified approach to directly and synergistically address the basic pathogenic processes involved in the progression of CKD. Isoquercetin has been added to reduce the side effect of the niacin flush while preserving niacin’s clinical effectiveness. It is our hope that the results of this study will be consistent with our previous observations of reversing stages of CKD in patients using the combination of immediate-release niacin, sodium bicarbonate, and calcium bicarbonate together. Here we describe our study design for evaluating the efficacy of the formulation for the treatment of stage 3 to 5 chronic kidney disease.
Objectives
The primary objective will be to assess whether there is a reversal of CKD stage, an increase in eGFR, and a reduction in urine albumin-creatinine ratio (UACR) after 3 months and 6 months of treatment when compared to the initial baseline. The average baseline mean values will be indexed to 100%. Changes will be calculated as the percent change from the baseline index of 100%. Secondary efficacy endpoints will consider serum phosphorus, serum bicarbonate, urine microalbumin, blood pressure, hemoglobin, serum calcium, vitamin D, intact parathyroid hormone, HDL, LDL, triglycerides, LpA, and ApoB. Exploratory efficacy endpoints may include additional measures of the mean change between baseline to 3 months of mortality, cardiovascular events, or other metrics.
Methods and Analysis
Eligibility Criteria
The study population will include patients with laboratory-supported, clinically diagnosed chronic kidney disease stage 3 to 5 as defined by an eGFR ranging from 10 to 45 mL/min/1.73 m2 that was measured on at least two occasions separated by at least 3 months. Alternatively, a UACR measure of greater than 300 will be considered as inclusion criteria.
Screening, Treatment Period, and End of Study
This will be an evaluation of routine clinical care where the formulation is added to the patient’s clinical regimen. The data from 100 or more patients will be deidentified as a limited data set. As a retrospective analysis of a deidentified database, this research will be exempt from an institutional review board under 45 Code of Federal Regulations 46.101(b). Accordingly, the need for patient consent will be waived. This is more of an evaluation of a quality improvement study than a clinical trial. Other clinical laboratory metrics may include, but are not limited to, measures of serum phosphorus, creatinine, protein (microalbumin), Cystatin C (CysC), CysC/glomerular filtration ratio, hemoglobin, blood pressure, bicarbonate, serum calcium, vitamin D, intact parathyroid hormone, and a full lipid profile. Treatment will then be initiated. Serum samples will be collected at 3 months and 6 months after treatment for analysis of eGFR and UACR (Figure 1). Secondary outcomes will also be considered.
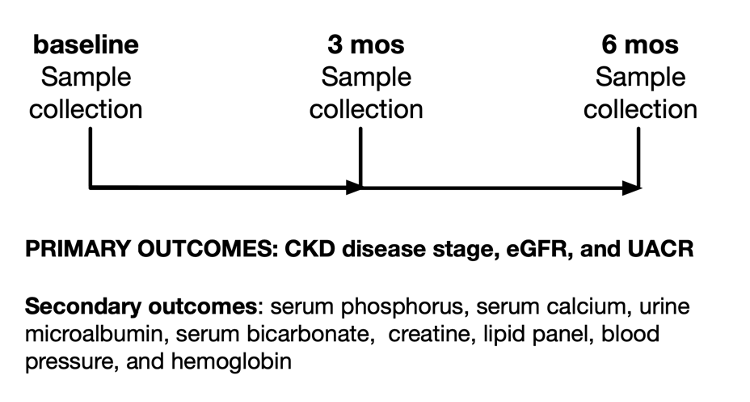
Figure 1. Pre- and post-study design for evaluating efficacy of the formulation in addressing chronic kidney disease.
Data Analysis and Statistical Methods
The primary endpoint, CKD stage with the mean change in eGFR and UACR will be analyzed by comparing it to baseline after 3 months and/or 6 months of treatment. Estimates of least-square means, standard errors, and 95% confidence intervals will be presented compared to baseline. The least square mean difference, the standard error of the difference, and the 95% confidence interval of the difference will also be presented.
CKD stages will be assessed based on the definitions (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, 2024):
- Stage 1: Kidney damage with normal or increased GFR (>90 mL/min/1.73 m2)
- Stage 2: Mild reduction in GFR (60-89 mL/min/1.73 m2)
- Stage 3a: Moderate reduction in GFR (45-59 mL/min/1.73 m2)
- Stage 3b: Moderate reduction in GFR (30-44 mL/min/1.73 m2)
- Stage 4: Severe reduction in GFR (15-29 mL/min/1.73 m2)
- Stage 5: Kidney failure (GFR < 15 mL/min/1.73 m2 or dialysis)
Dissemination
The data generated from this study will be presented in a follow-up publication in a timely manner. All final peer-reviewed manuscripts that result from this proposal will be submitted to PubMed Central and published in open-access journals when feasible. The study protocol and statistical analysis will be made publicly available. Participant level data will not be shared.
Discussion
Our approach directly addresses the basic processes causing CKD pathogenesis: hyperphosphatemia (niacin and calcium carbonate), metabolic acidosis (sodium bicarbonate), and proteinuria (niacin). The ingredients in the formulation have been independently proven in clinical trials to confer exceptional benefits when treating CKD. In the authors’ experience to date, we have personally observed over 25 reversals of CKD by multiple phases when recommending the use of simple TID niacin, BID sodium bicarbonate, and QD calcium bicarbonate (McConnell & Penberthy, 2021). In the proposed study, we have simplified this approach to a single pill taken TID. Based on our observations to date, we expect we will see similar positive outcomes.
The financial impact of CKD when it progresses to end-stage renal disease (dialysis-dependence; ESRD) can be devastating to the individual and also has a major impact on Medicare. Annual expenses for dialysis patients can vary widely, ranging from $720,000 to $2.2 million, with the financial impact of out-of-pocket expenses for CKD being more significant than that for cancer and stroke (Small, 2017). In 2020, Medicare spending for individuals with CKD surpassed $50 billion, which accounts for approximately 23.5% of the total Medicare fee-for-service spending (Francis, 2024). Medicare reportedly saves around $250,000 for every CKD patient who does not progress to ESRD (Golestaneh, 2017). All of these costs have the potential to be significantly mitigated through daily supplementation.
The earlier CKD is addressed, the more likely it is that patients can prevent an early death from cardiovascular complications. The United States has the second highest incidence of ESRD per capita in the world, making it crucial to address CKD at an early stage (Jha, 2013). CKD frequently develops with aging, affecting 68% of Americans aged 60 and older (Kidney Disease Statistics for the United States | NIDDK, n.d.). Notably, kidney disease ranks as the 9th leading cause of death in the United States (FastStats, 2021). The prevalence of advanced CKD has been increasing due to increases in type 2 diabetes, hypertension, and a low detection rate for the early stages of CKD (Jha, 2013). It makes the most impactful sense to address CKD at stage 3 or higher to prevent a significantly greater increase in CVD risk.
Niacin’s remarkable ability to address CKD speaks volumes to the positive outcomes observed repeatedly when using niacin to treat CVD, especially when one considers that CKD is a common diagnosis preceding death by CVD. Given that IR-niacin is now proven to be effective in treating both CKD and CVD, it comes as little surprise from our perspective that the Coronary Drug Project (1975-) had yielded quite possibly the most positive outcomes in the history of CVD randomized clinical trials (Berge & Canner, 1991; Canner, 1986)
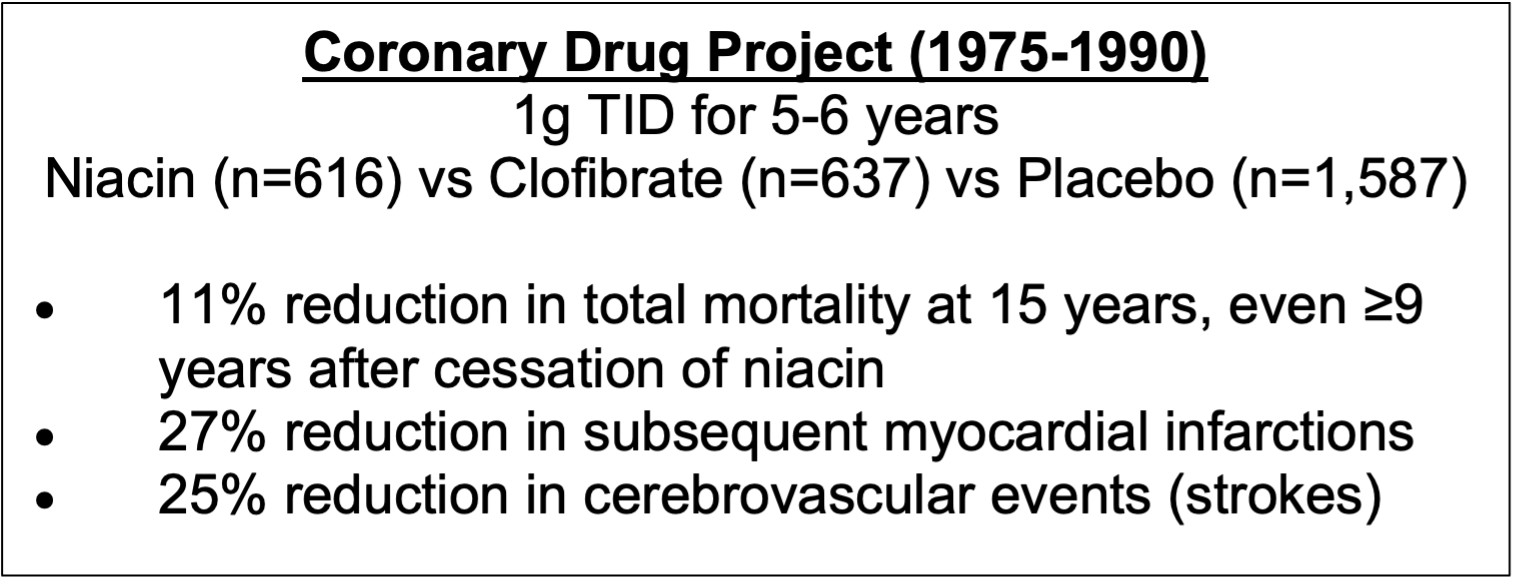
Figure 2. Coronary Drug Project (CDP) outcomes.
Furthermore, a meta-analysis of 11 randomized controlled trials involving 2682 patients in the active group versus 3934 in the control group determined that niacin reduced major coronary events by 25%, stroke by 26%, and any cardiovascular events by 27% (Bruckert, 2010). These exceptionally positive results from the use of niacin in CVD clinical trials may have been achieved largely due to niacin/nicotinic acid’s effects on optimizing kidney function when using niacin, particularly IR-niacin as was used in the CDP.
The flush response of IR-niacin is clearly a therapeutically beneficial activity, but it should be introduced and controlled to avoid unpleasantness or panic. Despite more than 119 clinical trials involving some form of niacin and counting in CVD clinical research, the results of IR-niacin in CDP have not been surpassed (D’Andrea, 2019). Unfortunately, many of the more modern trials have used inferior versions of niacin that were designed to reduce or eliminate the flush, but these did not yield outcomes as positive as the original IR-niacin (Dalton & Berry, 1992; D’Andrea, 2019; Penberthy, 2014; Schandelmaier, 2017). This has only been useful in increasing our understanding of the flush response and is requirement for many of the benefits starting with lipodystrophy, but it is now known to have more benefits for muscle growth and beyond (Carlson, 2005; Pirinen, 2020).
Basic science studies have now shown that the IR-niacin flush inhibits the progression of atherosclerosis and corrects lipodystrophy through the activation of the GPR109A receptor pathway in immune cells (Lukasova, 2011). Ultimately, these trials have proven that the flush response associated with IR-niacin is indeed beneficial for the treatment of CVD, which aligns with our observations and is the reason we have chosen to use IR-niacin in the formulation. We expect that this simple 1-pill formulation will demonstrate real-world benefits upon completion of this study.
Conflict of Interest
WTP, SM, RC, and CHF disclose intellectual property and other financial interests in Health Defender, LLC.
References
Al-Amrani, F., Al-Thihli, K., Al-Ajmi, E., Al-Futaisi, A., & Al-Murshedi, F. (2024). Transient response to high-dose niacin therapy in a patient with NAXE deficiency. JIMD Reports, 65(4), 212–225. https://doi.org/10.1002/jmd2.12425
Baking Soda May Slow Progression of Chronic Kidney Disease. (n.d.). Medscape. Retrieved September 30, 2021, from http://www.medscape.com/viewarticle/706043
Berge, K. G., & Canner, P. L. (1991). Coronary drug project: Experience with niacin. Coronary Drug Project Research Group. European Journal of Clinical Pharmacology, 40 Suppl 1, S49-51.
Berns, J. S. (2008). Editorials: Niacin and Related Compounds for Treating Hyperphosphatemia in Dialysis Patients. Seminars in Dialysis, 21(3), 203–205. https://doi.org/10.1111/j.1525-139X.2008.00426.x
Braidy, N., Lim, C. K., Grant, R., Brew, B. J., & Guillemin, G. J. (2013). Serum nicotinamide adenine dinucleotide levels through disease course in multiple sclerosis. Brain Research, 1537, 267–272. https://doi.org/10.1016/j.brainres.2013.08.025
Bruckert, E., Labreuche, J., & Amarenco, P. (2010). Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis, 210(2), 353–361. https://doi.org/10.1016/j.atherosclerosis.2009.12.023
Canner, P. L., Berge, K. G., Wenger, N. K., Stamler, J., Friedman, L., Prineas, R. J., & Friedewald, W. (1986). Fifteen year mortality in Coronary Drug Project patients: Long-term benefit with niacin. Journal of the American College of Cardiology, 8(6), 1245–1255. https://doi.org/10.1016/s0735-1097(86)80293-5
Carlson, L. A. (2005). Nicotinic acid: The broad-spectrum lipid drug. A 50th anniversary review. Journal of Internal Medicine, 258(2), 94–114. https://doi.org/10.1111/j.1365-2796.2005.01528.x
Chanvillard, L., Tammaro, A., & Sorrentino, V. (2022). NAD+ Metabolism and Interventions in Premature Renal Aging and Chronic Kidney Disease. Cells, 12(1), 21. https://doi.org/10.3390/cells12010021
Chronic Kidney Disease. (n.d.). Cleveland Clinic. Retrieved July 30, 2024, from https://my.clevelandclinic.org/health/diseases/15096-chronic-kidney-disease
Csiszar, A., Tarantini, S., Yabluchanskiy, A., Balasubramanian, P., Kiss, T., Farkas, E., Baur, J. A., & Ungvari, Z. (2019). Role of endothelial NAD+ deficiency in age-related vascular dysfunction. American Journal of Physiology. Heart and Circulatory Physiology, 316(6), H1253–H1266. https://doi.org/10.1152/ajpheart.00039.2019
Dalton, T. A., & Berry, R. S. (1992). Hepatotoxicity associated with sustained-release niacin. The American Journal of Medicine, 93(1), 102–104. https://doi.org/10.1016/0002-9343(92)90689-9
D’Andrea, E., Hey, S. P., Ramirez, C. L., & Kesselheim, A. S. (2019). Assessment of the Role of Niacin in Managing Cardiovascular Disease Outcomes: A Systematic Review and Meta-analysis. JAMA Network Open, 2(4), e192224. https://doi.org/10.1001/jamanetworkopen.2019.2224
de Brito-Ashurst, I., Varagunam, M., Raftery, M. J., & Yaqoob, M. M. (2009). Bicarbonate supplementation slows progression of CKD and improves nutritional status. Journal of the American Society of Nephrology: JASN, 20(9), 2075–2084. https://doi.org/10.1681/ASN.2008111205
FastStats. (2021, April 9). https://www.cdc.gov/nchs/fastats/deaths.htm
Francis, A., Harhay, M. N., Ong, A. C. M., Tummalapalli, S. L., Ortiz, A., Fogo, A. B., Fliser, D., Roy-Chaudhury, P., Fontana, M., Nangaku, M., Wanner, C., Malik, C., Hradsky, A., Adu, D., Bavanandan, S., Cusumano, A., Sola, L., Ulasi, I., & Jha, V. (2024). Chronic kidney disease and the global public health agenda: An international consensus. Nature Reviews Nephrology, 20(7), 473–485. https://doi.org/10.1038/s41581-024-00820-6
Golestaneh, L., Alvarez, P. J., Reaven, N. L., Funk, S. E., McGaughey, K. J., Romero, A., Brenner, M. S., & Onuigbo, M. (2017). All-cause costs increase exponentially with increased chronic kidney disease stage. The American Journal of Managed Care, 23(10 Suppl), S163–S172.
Jankowski, J., Floege, J., Fliser, D., Böhm, M., & Marx, N. (2021). Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation, 143(11), 1157–1172. https://doi.org/10.1161/CIRCULATIONAHA.120.050686
Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., Saran, R., Wang, A. Y.-M., & Yang, C.-W. (2013). Chronic kidney disease: Global dimension and perspectives. Lancet (London, England), 382(9888), 260–272. https://doi.org/10.1016/S0140-6736(13)60687-X
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. (2024). KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International, 105(4S), S117–S314. https://doi.org/10.1016/j.kint.2023.10.018
Kidney Disease Statistics for the United States | NIDDK. (n.d.). National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved September 19, 2021, from https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease
Lautrup, S., Hou, Y., Fang, E. F., & Bohr, V. A. (2024). Roles of NAD+ in Health and Aging. Cold Spring Harbor Perspectives in Medicine, 14(1), a041193. https://doi.org/10.1101/cshperspect.a041193
Lukasova, M., Malaval, C., Gille, A., Kero, J., & Offermanns, S. (2011). Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. The Journal of Clinical Investigation, 121(3), 1163–1173. https://doi.org/10.1172/JCI41651
McConnell, S., & Penberthy, W. T. (2021). Reversing Chronic Kidney Disease with Niacin and Sodium Bicarbonate. Orthomolecular Medicine News Service.
Migliavacca, E., Tay, S. K. H., Patel, H. P., Sonntag, T., Civiletto, G., McFarlane, C., Forrester, T., Barton, S. J., Leow, M. K., Antoun, E., Charpagne, A., Seng Chong, Y., Descombes, P., Feng, L., Francis-Emmanuel, P., Garratt, E. S., Giner, M. P., Green, C. O., Karaz, S., … Feige, J. N. (2019). Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nature Communications, 10(1), 5808. https://doi.org/10.1038/s41467-019-13694-1
Nicotinic Acid and Nicotinamide: A Review of Their Use for Hyperphosphatemia in Dialysis Patients—Rennick—2013—Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy—Wiley Online Library. (n.d.). Retrieved September 30, 2021, from https://accpjournals.onlinelibrary.wiley.com/doi/abs/10.1002/phar.1258
Rao M, Steffes M, Bostom A, Ix JH. (2014) Effect of niacin on FGF23 concentration in chronic kidney disease. Am J Nephrol 39, 484-490. https://pubmed.ncbi.nlm.nih.gov/24854458
Penberthy, W. T. (2014). Laropiprant is the Bad One; Niacin is/was/will always be the Good One. Orthomolecular Medicine News Service. https://www.orthomolecular.org/resources/omns/v10n12.shtml
Pirinen, E., Auranen, M., Khan, N. A., Brilhante, V., Urho, N., Pessia, A., Hakkarainen, A., Kuula, J., Heinonen, U., Schmidt, M. S., Haimilahti, K., Piirilä, P., Lundbom, N., Taskinen, M.-R., Brenner, C., Velagapudi, V., Pietiläinen, K. H., & Suomalainen, A. (2020). Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metabolism, 31(6), 1078-1090.e5. https://doi.org/10.1016/j.cmet.2020.04.008
Poljšak, B., Kovač, V., Špalj, S., & Milisav, I. (2023). The Central Role of the NAD+ Molecule in the Development of Aging and the Prevention of Chronic Age-Related Diseases: Strategies for NAD+ Modulation. International Journal of Molecular Sciences, 24(3), 2959. https://doi.org/10.3390/ijms24032959
Rai, N. K., Wang, Z., Drawz, P. E., Connett, J., & Murphy, D. P. (2023). CKD Progression Risk and Subsequent Cause of Death: A Population-Based Cohort Study. Kidney Medicine, 5(4), 100604. https://doi.org/10.1016/j.xkme.2023.100604
Schandelmaier, S., Briel, M., Saccilotto, R., Olu, K. K., Arpagaus, A., Hemkens, L. G., & Nordmann, A. J. (2017). Niacin for primary and secondary prevention of cardiovascular events. The Cochrane Database of Systematic Reviews, 6(6), CD009744. https://doi.org/10.1002/14651858.CD009744.pub2
Small, C., Kramer, H. J., Griffin, K. A., Vellanki, K., Leehey, D. J., Bansal, V. K., & Markossian, T. W. (2017). Non-dialysis dependent chronic kidney disease is associated with high total and out-of-pocket healthcare expenditures. BMC Nephrology, 18(1), 3. https://doi.org/10.1186/s12882-016-0432-2
Song, Z., Park, S. H., Mu, W.-C., Feng, Y., Wang, C.-L., Wang, Y., Barthez, M., Maruichi, A., Guo, J., Yang, F., Lin, A. W., Heydari, K., Chini, C. C. S., Chini, E. N., Jang, C., & Chen, D. (2024). An NAD+-dependent metabolic checkpoint regulates hematopoietic stem cell activation and aging. Nature Aging. https://doi.org/10.1038/s43587-024-00670-8
Watanabe H, et al. (2021) Inhibition of the renin-angiotensin system causes concentric hypertrophy of renal arterioles in mice and humans. JCI Insight.6, e154337 (2021).


