Introduction
Throughout human evolutionary history, our interaction with both primate ancestors and domesticated animals has resulted in the acquisition of approximately 300 species of parasitic worms (Król et al., 2019) and over 70 species of protozoa (Cox, 2002). Ancient fecal samples have yielded evidence of nearly all known parasites specific to humans (Araújo et al., 2003).
A parasite is an organism that lives in or on another species, known as its host, deriving nutrients at the host’s expense, thus causing what is commonly referred to as a parasitic infection (Forman & Maryanti, 2021). Parasitology traditionally limits its focus to protozoa (Imam, 2009). T, helminths (McVeigh, 2020), arthropods (Di Giovanni et al., 2021), and their vector species, although it is entirely proper from a biological standpoint to classify bacteria, fungi, and viruses as parasites (Bogitsh, Carter, & Oeltmann, 2013).
Parasitic Infections: A Global Health Challenge
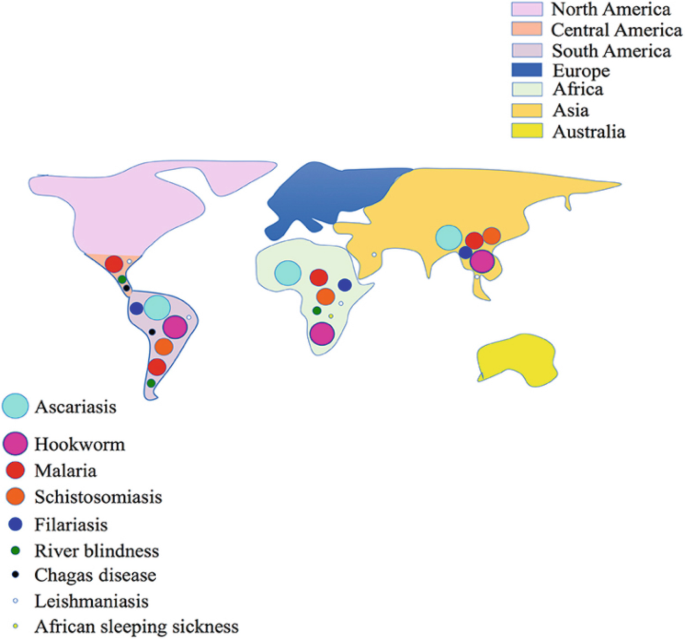
Human parasitic infections represent a significant public health burden worldwide, particularly in tropical and subtropical regions (Ung et al., 2021; Bogitsh, Carter, & Oeltmann, 2013). These infections are caused by various protozoa, helminths, and ectoparasites, leading to a range of clinical manifestations from mild discomfort to severe morbidity and mortality (Cox, 2002). Some of the most common parasitic diseases include malaria, schistosomiasis, lymphatic filariasis, soil- transmitted helminthiasis, and leishmaniasis (Ung et al. 2021).
Figure 1. Global Parasitic Disease Epidemiology. Adapted from Nag & Kalita, (2022)
A Clinical Overview of Common Parasitic Diseases
Malaria, caused by Plasmodium parasites and transmitted through the bite of infected mosquitoes, remains a major cause of morbidity and mortality worldwide, particularly in sub-Saharan Africa (Bogitsh, Carter & Oeltmann, 2013). Despite significant progress in control efforts, including the distribution of insecticide-treated bed nets and antimalarial drugs, challenges such as insecticide resistance and limited access to healthcare continue to hinder eradication efforts (Ung et al. 2021).
Schistosomiasis, transmitted through contact with contaminated water inhabited by freshwater snails carrying Schistosoma parasites, affects over 200 million people globally, primarily in tropical and subtropical regions (Mutuku, 2020). The disease can lead to chronic complications such as liver and spleen enlargement, bladder cancer, and kidney damage, contributing to the cycle of poverty and economic instability in affected communities (Ung et al. 2021).
Lymphatic filariasis, caused by filarial worms transmitted through the bites of infected mosquitoes, affects over 120 million people worldwide, causing severe disability and disfigurement. The disease can lead to lymphedema, elephantiasis, and hydrocele, significantly impacting the quality of life of affected individuals and imposing a considerable economic burden on endemic countries (Ung et al. 2021).
Soil-transmitted helminthiasis, including infections with roundworms, whipworms, and hookworms, affects over 1.5 billion people globally, particularly in areas with poor sanitation and hygiene practices. These parasites thrive in warm and humid environments and can lead to malnutrition, anemia, and impaired cognitive development, particularly in children (Ung et al. 2021).
Leishmaniasis, caused by Leishmania parasites transmitted through the bite of infected sandflies, manifests in various clinical forms ranging from cutaneous lesions to visceral involvement, depending on the species of parasite and host immune response. The disease affects millions of people worldwide, with an estimated 350 million people at risk of infection in endemic regions (Ung et al. 2021).
Conventional treatment approaches often rely on antiparasitic drugs, which may be associated with limitations such as drug resistance (Pink et al., 2005), toxicity (Rosenblatt, 1992), and cost (Shahriar, & Alpern, 2020).
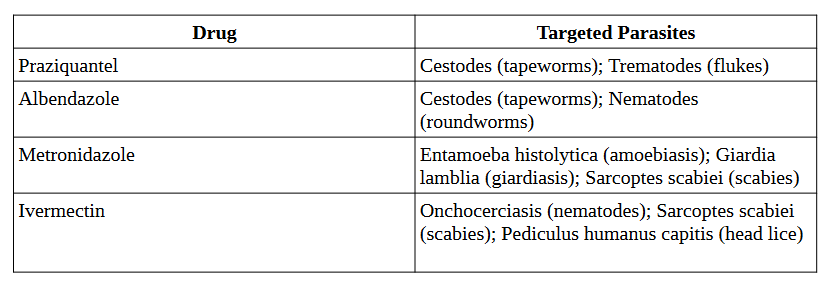
Classic antiparasitic drugs, including chloroquine for malaria, metronidazole for amoebiasis, and praziquantel for helminthic infections are extensively discussed in the review by Campbell and Soman-Faulkner (2023).
The World Health Organization (WHO) recognizes 36 antiparasitic drugs as essential, underscoring their critical role in global health initiatives (Arete-Zoe, 2017). Synthetic drugs are commonly used to treat parasitic diseases, but exploring plant-based compounds presents an intriguing avenue for potential advancements in treatment.
A review by Ranasinghe et al. (2023) evaluated 507 plant species, primarily from the Fabaceae, Asteraceae, Combretaceae, and Lamiaceae families, for their antiparasitic effects against gastrointestinal parasites, with a focus on organisms such as Entamoeba histolytica and Giardia duodenalis; ninety-one plant species and thirty-four compounds were identified as demonstrating significant in vitro efficacy against parasites.
Table 1. Antiparasitic Drugs and Their Targeted Parasites (Campbell and Soman-Faulkner, 2023)
Nutrition significantly influences susceptibility to all parasitic infections (Coop, & Kyriazakis, 1999). Malnutrition, especially protein deficiency, heightens vulnerability and exacerbates conditions such as hookworm infection – leading to anemia, weight loss, abdominal swelling, and mental fatigue (Bogitsh, Carter, & Oeltmann, 2013).
Poor nutrition not only increases susceptibility to parasitic infections but also can be both a consequence and exacerbating factor of infection-induced malnutrition. Onchocerciasis, the second leading infectious cause of blindness, affects 20 million people, mainly in tropical Africa, with another 120 million at risk. Caused by Onchocerca volvulus, it leads to “river blindness” and severe dermatitis, resembling vitamin A deficiency symptoms , suggesting a possible competition for or interference with vitamin A metabolism in the host (Bogitsh, Carter, & Oeltmann, 2013). De Gier et al. (2014) analyzed 37 studies on Helminth infections and micronutrients in school-age children and found that helminth infections correlate with decreased serum retinol but not ferritin levels in children, concluding that further research on other micronutrients is needed.
The Potential Role of Orthomolecular Medicine in Addressing Parasitic Infections
Orthomolecular medicine, as defined by Pauling in 1968, focuses on restoring and maintaining health through the administration of substances naturally present in the body. The aging process, often accelerated by factors like free radical exposure (Migliore & Coppedè, 2009), inflammation (Bektas, et al, 2018), and toxic exposures (Dutta, et al., 2023), can be slowed or reversed through orthomolecular therapy, alongside addressing health issues (Carter, 2019).
Orthomolecular medicine can potentially contribute to the One Health approach by emphasizing optimal nutrition and natural substances to boost immune function, support host resistance, and reduce parasite burden in both humans and animals, thus complementing efforts to control parasitic diseases while promoting overall health and well-being. Global aging poses a multifaceted challenge within the One Health paradigm. Aging, influenced by a multitude of interconnected factors such as bio-genetics, environment, and socioeconomic forces, not only manifests internal vulnerabilities like frailty and comorbidities but also external challenges such as social isolation and financial strain, collectively rendering the elderly population more susceptible to infections, including parasitic infections (Forman & Maryanti, 2021).
Scientific studies increasingly support the therapeutic and preventive benefits of high doses of nutrients. Vitamins C and E, beta-carotene, B-complex vitamins, and coenzyme Q10 have demonstrated positive effects on health and longevity at doses exceeding the Recommended Dietary Allowance (RDA). Although mineral requirements, such as magnesium, zinc, and chromium, are closer to the RDA, supplements beyond dietary intake levels may still be necessary for disease prevention, treatment, and slowing the aging process (Carter, 2019).
Orthomolecular medicine offers a potential alternative therapy for parasitic infections. By restoring health through the administration of natural substances present in the body (Hemat, 2004), orthomolecular medicine can address underlying health issues while slowing the aging process (Carter, 2019). High-dose nutrient supplementation, a cornerstone of orthomolecular therapy, (Cathcart, Cott & Foster 2014) has shown promising therapeutic and preventive benefits in various health conditions. However, its efficacy in treating parasitic infections at orthomolecular doses remains largely unexplored.
The immune response to parasites involves a complex interplay of defense mechanisms. Nitric oxide (NO) plays a crucial role by targeting cysteine proteases essential for parasite life cycles and host-parasite interactions (Ascenzi et al., 2003). Histones, known for DNA regulation, also act as key mediators of host defense, triggering inflammatory responses and directly combating parasites (Hoeksema et al., 2016).
Additionally, a robust IFN-γ response in humans indicates effective pro-inflammatory action against parasites (Artavanis-Tsakonas et al., 2003). Despite variations among parasite groups, common immune reactions are activated upon infection, involving pattern recognition, inflammatory signaling, effector molecule expression, antigen presentation, and establishment of adaptive immune responses, contributing to infection control (Buchmann, 2022). This orchestrated immune response highlights the host’s intricate mechanisms to combat parasitic challenges.
Huemer (2006), while exploring the orthomolecular ramifications of chronic renal disease, highlights the importance of mega doses of vitamins B6, B12, folate, and trimethyl glycine (betaine) in increasing nitric oxide levels by inhibiting homocysteine. Short-term supplementation with 750 mg of vitamin E leads to increased production of the T helper 1 cytokine IFN-gamma (Malmberg et al., 2002; Saul, 2003).
The effectiveness of vitamin C to enhance the innate immune response is well established (Hoang et al, 2020), and high-dose vitamin C showed a non-significant trend towards increased cell- mediated immune responses in healthy elderly individuals (Goodwin et al, 1983). According to Mikirova (2020), continuous ascorbate infusions may stimulate histone function, potentially influencing gene expression.
Recent clinical trials indicate that vitamin A supplementation reduces morbidity and mortality in various infectious diseases, while studies in animal models and cell lines highlight its significant role in immunity, including modulation of mucins and keratins expression, lymphopoiesis, apoptosis, cytokine expression, antibody production, and the function of immune cells such as neutrophils, natural killer cells, monocytes, macrophages, T lymphocytes, and B lymphocytes (Semba, 2007; Ash, 2011).
The intricate interplay between molecular targets and nutrient interactions underscores the plausibility of these interventions in effectively combating human parasitosis. Orthomolecular medicine, focusing on high-dose nutrient supplementation, potentially offers an adjuncive therapy for parasitic infections, but its efficacy remains largely unexplored in this context.
Orthomolecular Interventions
In addition to the molecularly targeted interventions of vitamins A, B, C, and E, several nutrients have emerged as promising candidates in clinical trials for combating parasitic infections and their complications.
Iron
Iron supplementation is recommended to address potential anemia associated with infections caused by Ancylostoma duodenale and Necator americanus, even before diagnosis or treatment initiation (Kucik, Martin, & Sortor, 2004).
Zinc
Kucik, Martin, and Sortor (2004) showed that parasites are better able to survive in zinc-deficient hosts compared to well-nourished hosts. Zinc deficiency, affects gut immunity, prolonging parasite survival (Scott & Koski, 2000). Further, zinc supplementation, particularly with zinc gluconate, has shown promising evidence of reducing Plasmodium falciparum-mediated febrile episodes in malaria (Overbeck, Rink, & Haase, 2008). Kotepui et al. (2023) conducted a systematic review on the impact of daily oral zinc supplementation, either alone or in combination with other nutrients, on malaria risk. They found no significant effect of zinc alone but suggested a potential benefit when combined with other micronutrients. This underscores the necessity for larger studies to clarify the effects of multi-nutrient supplementation on malaria risk.
Folate
Clinical trials investigating folate’s impact on malaria progression have primarily focused on antimalarial drug efficacy rather than direct folate intake, making it challenging to disentangle the specific effects of folate supplementation on malaria risk (Nzila, Okombo, & Hyde, 2016).
Vitamin B1
Vitamin B1 (thiamine) deficiency reduces resistance to parasitic infestations in rats, highlighting its crucial role in immune function against helminth infections (Watt, 1944). Children who did not meet the recommended intake for thiamin had a higher prevalence of infection with Trichuris trichiura suggesting a potential association between thiamin deficiency and susceptibility to parasitic infections (Papier et al., 2014).
Vitamin B12
Layden et al. (2018) conducted a review on the interplay of neglected tropical diseases (NTDs) and vitamin B12 (cobalamin) highlighting the scarcity of literature and the need for future prospective studies to establish the role of vitamin B12 in NTD etiology and potential clinical significance.
Vitamin C
Vitamin C exhibits potent antiparasitic effects against Trypanosoma cruzi, potentially through a pro-oxidant mechanism, making it a promising candidate for Chagas’ disease treatment (Puente et al., 2018). Klenner (1954) suggests the use of high-dose intravenous vitamin C, alongside para- aminobenzoic acid, for treating trichinosis, advocating daily injections of four to twelve grams of vitamin C for non-responsive patients due to its roles in antibody formation and detoxification.
Vitamin D
Vitamin D deficiency is associated with increased susceptibility to infectious diseases, including tuberculosis and Leishmania parasitic infections, while sufficient levels have been shown to enhance immune responses against pathogens such as Mycobacterium tuberculosis and Campylobacter jejuni (Zughaier, Lubberts, & Bener, 2020). Vitamin D has potential as an adjunctive therapy in parasitic diseases like leishmaniasis, owing to its modulation of inflammation and wound healing pathways (Ramos-Martínez et al., 2015).
Arachidonic Acid
Arachidonic acid (ARA) has shown potent schistosomicidal effects by inducing parasite death through excessive hydrolysis of sphingomyelin (SM) and has demonstrated efficacy in both in vitro and in vivo studies against Schistosoma mansoni and Schistosoma haematobium infections (Tallima, Hanna, & El Ridi, 2020).
Research Gap and Objectives
The evidence supporting the efficacy of orthomolecular medicine in treating human parasites remains limited and inconclusive. This review seeks to systematically evaluate the available literature to elucidate the role of orthomolecular interventions in the management of human parasitic infections.
Orthomolecular interventions can enhance host immune responses, inhibiting parasite growth. Research supporting the therapeutic and preventive benefits of high doses of nutrients exists, but their efficacy in treating parasitic infections at the appropriate doses remains uncertain.
While our analysis encompassed a comprehensive search of relevant studies, it is noteworthy that not a single study meeting the criteria for orthomolecular dosing was identified. This absence of empirical data underscores a critical gap in our understanding of the potential efficacy of such interventions in this context.
Despite this limitation, our review provides valuable insights into the current state of research and highlights the need for further investigation into the use of orthomolecular medicine as a therapeutic approach for parasitic infections. By elucidating existing gaps in the literature, our study aims to inform future research endeavors and contribute to the advancement of effective treatment strategies for parasitic diseases.
Methods
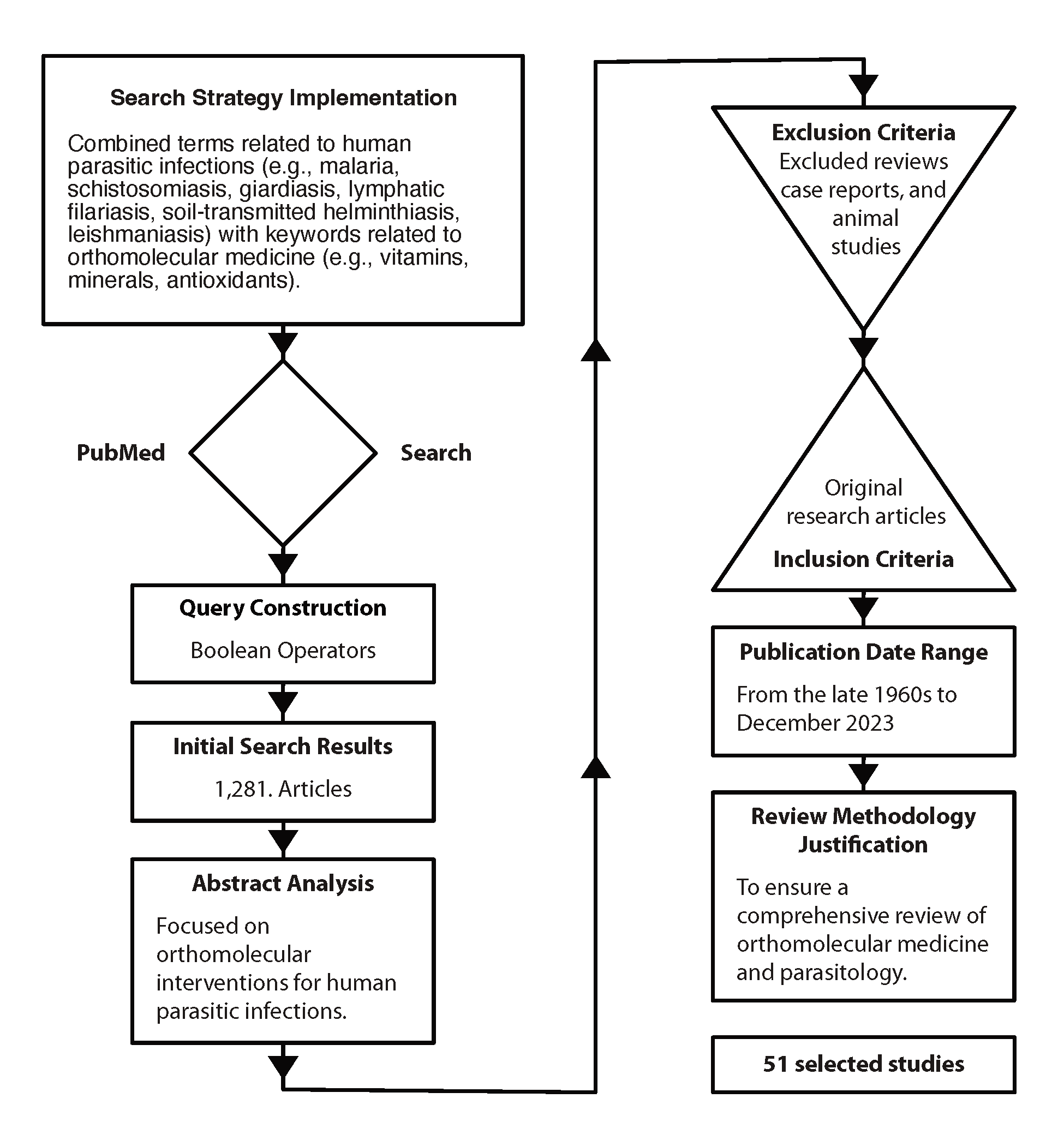
A meticulous search strategy was implemented using PubMed, a renowned biomedical database, to identify relevant literature on orthomolecular medicine’s efficacy in treating human parasitic infections published up to December 2023. The search query covered two domains: human parasitic infections, and orthomolecular medicine. Terms for parasitic infections like malaria, schistosomiasis, and giardiasis were included to ensure comprehensive coverage. Similarly, terms for orthomolecular medicine, such as vitamins, minerals, and antioxidants, were selected to encapsulate its essence.
Boolean operators, namely OR and AND, were strategically utilized to refine the search and delineate logical relationships between terms. The resulting query, “(malaria OR schistosomiasis OR giardiasis) AND (orthomolecular medicine OR vitamins OR minerals OR antioxidants),” aimed to retrieve articles at the intersection of these domains. This systematic approach aimed to compile literature for a comprehensive review, providing insights into the role of orthomolecular medicine in combating human parasitic infections.
The initial search yielded 1,281 results, which were subsequently narrowed down to 51 through abstract analysis.
Table 2. Parasitic Infections & Orthomolecular Approaches: PubMed Search Terms
Literature Review Methodology
The exclusion of a significant number of studies from the initial search results can be attributed to the fact that many of them focused on pharmaco- or toxicomolecular interventions rather than orthomolecular medicine or the specific nutrients (vitamins, minerals, antioxidants) mentioned in the search criteria. Therefore, studies that did not align closely with the targeted interventions or topics were omitted during the abstract analysis phase, resulting in a smaller subset of relevant studies.
Studies were included if they met the following criteria:
- original research articles evaluating the efficacy of orthomolecular interventions (e.g., vitamin supplementation, mineral therapy) in treating human parasitic infections
- inclusion of clinical outcomes such as parasite clearance, symptom resolution, and adverse effects
- availability of sufficient data to calculate effect sizes or odds ratios.
- publication date spanning several decades, from the late 1960s to December 2023.
Studies were excluded if they were reviews, case reports, or animal studies. Excluding reviews, case reports, and animal studies from the analysis ensures the review focuses solely on high-quality, original research, thereby maintaining rigor by prioritizing studies with larger sample sizes, rigorous methodologies, and direct applicability to human populations in evaluating the efficacy of orthomolecular interventions for human parasitic infections.
Addressing potential bias in the review process itself involved several strategies to mitigate publication bias and selective outcome reporting. These include utilizing multiple databases to minimize publication bias, conducting thorough manual searches of reference lists, registering the review protocol to enhance transparency and reduce selective outcome reporting, and employing sensitivity analyses to assess the impact of potential bias on the review findings, ensuring a comprehensive and unbiased synthesis of the evidence.
The extended timeframe from the 1960s to December 2023 was chosen to capture the historical development of research in both orthomolecular medicine and parasitology, ensuring a comprehensive review of the field. The choice of the 1960s as the timeframe for parasitology is justified by its embeddedness in a longer historical context of continuous discovery and formalization, interdisciplinary nature of this research, and significant technological advancements during that era (Roberts, L. S., 2004).
A comprehensive search of relevant literature was conducted to evaluate the efficacy of orthomolecular interventions in treating human parasitic infections.
The review included original research articles meeting specific criteria related to orthomolecular interventions and clinical outcomes.
Figure 3. PubMed Search Strategy
Results
The analysis of the 51 selected studies revealed a diverse spectrum of research focused on nutritional interventions for human parasitic infections. Among these studies, the distribution of research varied across different nutrients and interventions.
Table 3. Nutrient and Intervention Analysis for Parasitic Infections: Study Counts

The tables in the appendix provide a comprehensive listing of the studies included in the review, organized by specific micronutrients, allowing for easy reference and examination of the primary research contributing to the analysis.
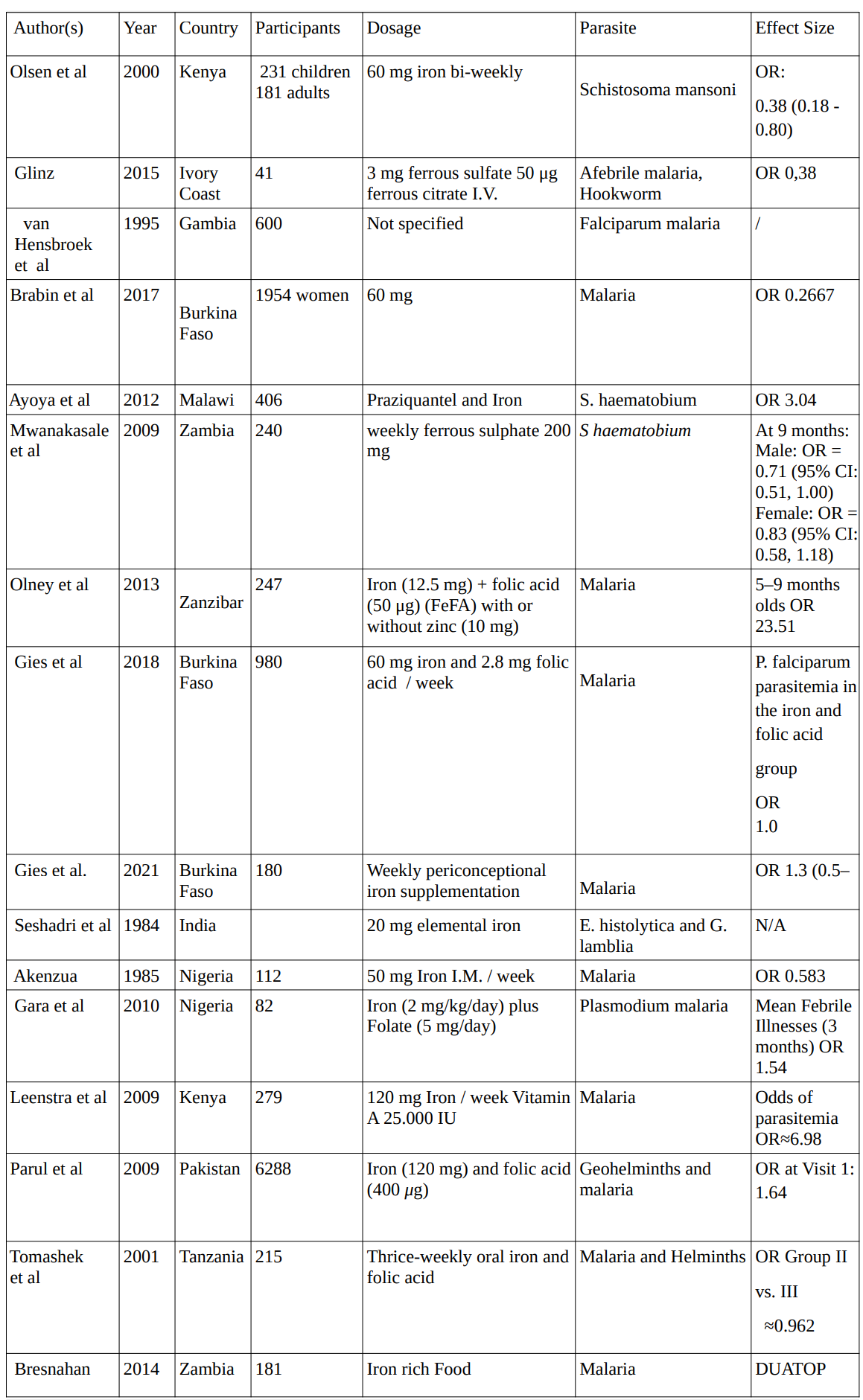
Iron
In 16 studies conducted between 1984 and 2021 investigating the effects of iron supplementation on various parasites, involving a total of 11,565 participants, the average dosage for iron supplementation administered across studies was approximately 48.75 mg.
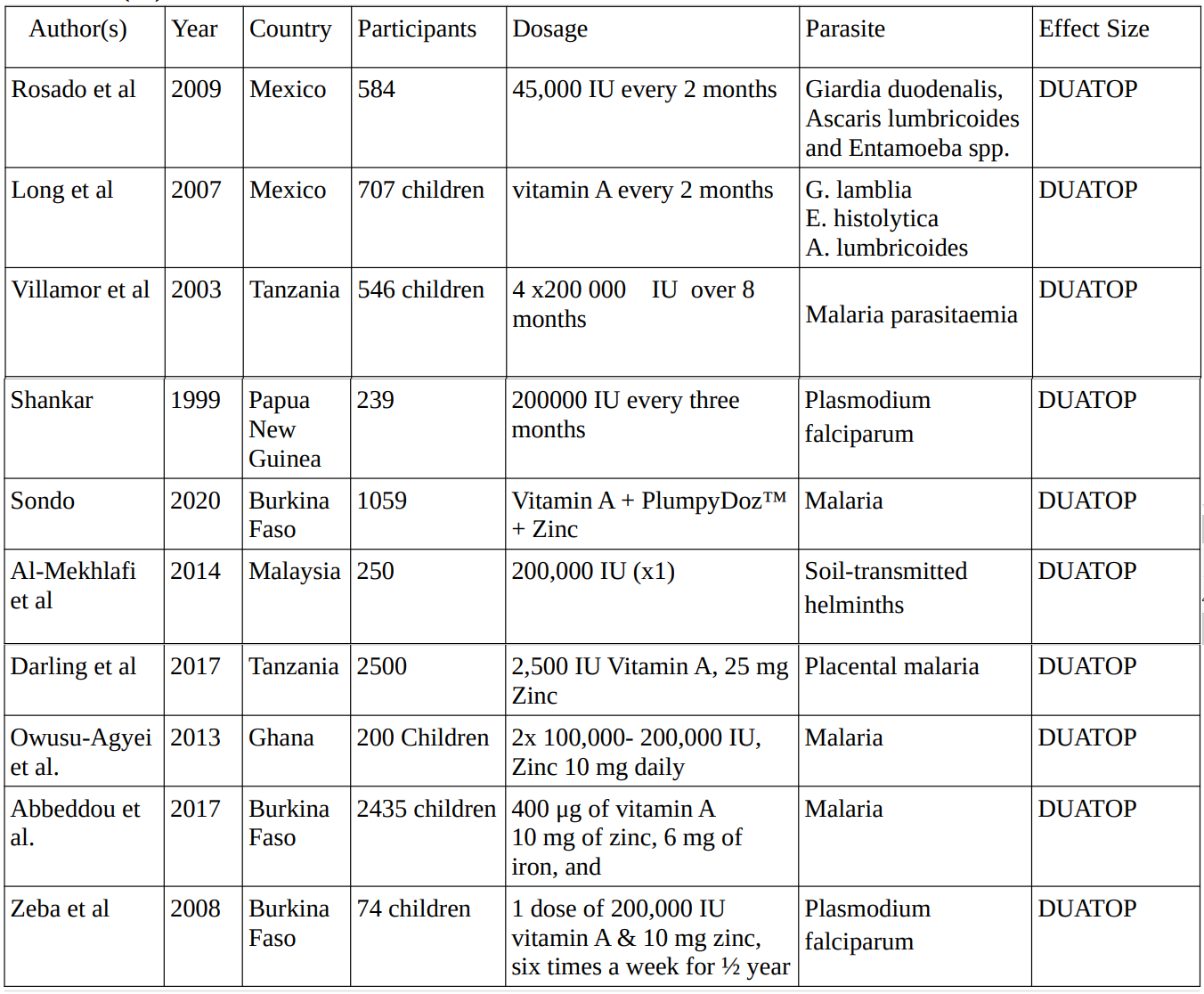
Vitamin A
In 9 studies conducted between 1999 and 2020 investigating the effects of vitamin A supplementation on various parasites, involving a total of 8,364 participants, the dosage for vitamin A supplementation administered across studies varied, ranging from 2,500 IU to 200,000 IU, with dosing frequency ranging from single doses to every three months.
Multivitamin
The 8 studies conducted between 2003 and 2019 investigating the effects of multivitamin supplementation on various parasites, involving a total of 13,844 participants, supplementation varied widely in composition and format, including unspecified combinations of iron, zinc, and calcium, vitamin B complex, vitamins C and E, as well as lipid-based nutrient supplements and micronutrient powders, with frequencies ranging from daily intake to weekly sachets.
Zinc
In the 6 studies conducted between 1969 and 2016 investigating the effects of zinc supplementation on various parasites, involving a total of 1,918 participants, supplementation ranged from daily doses of 10 mg to 60 mg of zinc sulfate or zinc methionine, with dosing frequency varying from daily intake to bi-weekly administration. These studies were targeting parasites such as Schistosoma hematobium, Ascaris lumbricoides, Giardia intestinalis, Entamoeba histolytica/E. dispar, malaria, cutaneous leishmaniasis, and helminths/protozoa.
Vitamin C
In the 2 studies conducted in Uganda in 2013 and Ghana in 2015 investigating the effects of vitamin C supplementation on parasites, involving a total of 2,983 participants, supplementation dosages ranged from 66 mg to 180 mg, administered via mango juice as a drink. These studies targeted schistosomes, malaria, and hookworm.
Arachidonic Acid
In the 2 studies conducted in Egypt in 2014 and 2015 investigating the effects of arachidonic acid supplementation on Schistosoma mansoni, involving a total of 334 participants, supplementation dosages ranged from 10 mg/kg to 396 mg.
Criteria for Orthomolecular Dosing
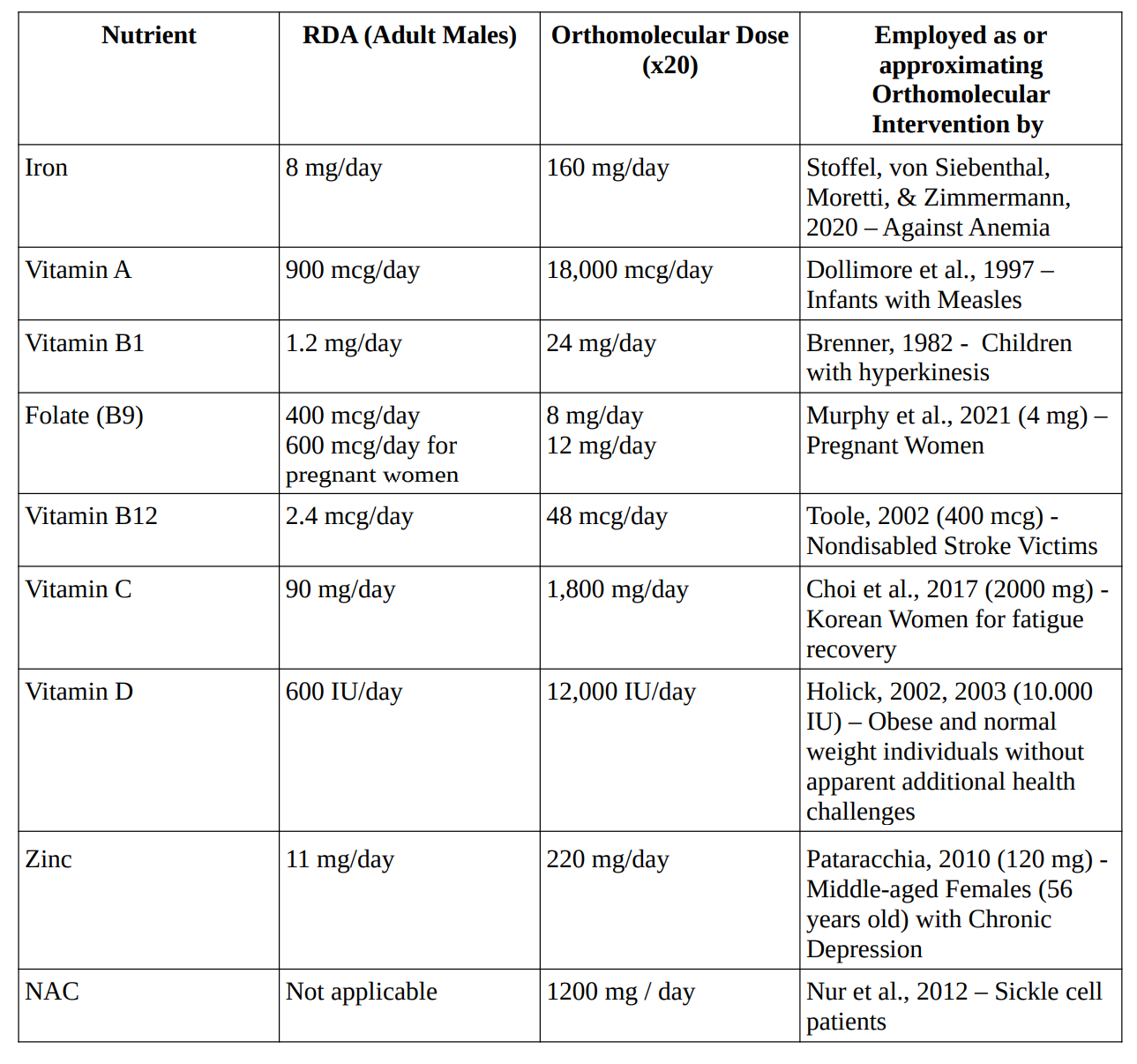
Hoffer (1976) defined a megadose as a dosage of 800 International Units (IU) or more of vitamin E per day, exceeding the Recommended Daily Allowance (RDA) of 33.3 IU by approximately 24 times. Megavitamin or orthomolecular doses were therefore defined as nutrient supplementation exceeding twenty times the recommended dietary allowances (RDAs) or daily intakes (RDIs) established by authoritative bodies such as the Institute of Medicine (IOM) or the World Health Organization (WHO).
For example, megavitamin doses of vitamin C were considered to be doses exceeding 2000 milligrams per day. Studies administering nutrients at or below these threshold doses were excluded from further analysis.
Safety Considerations
Critics of orthomolecular treatment express concerns about the potential risks associated with high-dose nutrient supplementation, highlighting that such doses may lead to adverse effects or interactions with medications, posing safety risks to patients (Hoffer, 1983).
Prousky (2013) extensively discussed the adverse effects of orthomolecular medicine, highlighting that Orthomolecular Medicine Therapy (OMT) is generally not associated with physiological dependence, withdrawal symptoms, or long-term harm, although there are instances where excessive doses of micronutrients can lead to adverse reactions. These adverse effects include hepatotoxicity and neurotoxicity, particularly with high doses of Vitamin B3/B6; however, it is emphasized that such adverse effects are usually self-limited and reversible upon reducing or discontinuing the dosage. Additionally, the therapeutic indexes (TIs) of orthomolecules tend to be extremely large, suggesting that the amount needed to produce a therapeutic effect is significantly lower than the amount needed to produce a lethal effect, thus implying a relatively low risk of mortality associated with OMT.
The table “Orthomolecular Doses Compared to Recommended Daily Allowances for Various Nutrients in Intervention Studies” lists studies in which high doses of the aforementioned nutrients were safely administered to human populations.
Table 4. Orthomolecular Doses Compared to Recommended Daily Allowances for Various Nutrients in Intervention Studies
Orthomolecular interventions may exert their effects through various molecular pathways, including immune modulation, antioxidant activity, and direct inhibition of parasite growth.
The included studies investigated a variety of nutritional interventions, including vitamin A, vitamin C, vitamin D, zinc, and selenium, alone or in combination with conventional antiparasitic drugs. However, it is important to note that none of the studies reached the orthomolecular doses mentioned earlier in the analysis.
The absence of studies meeting orthomolecular doses for parasitic infections is a significant limitation of the review. Despite conducting a comprehensive search and analysis of relevant literature, no studies were found that specifically evaluated the efficacy of orthomolecular interventions at doses considered orthomolecular for treating parasitic infections.
Discussion
The following limitations have several implications for the review findings:
- Lack of Direct EvidenceThe absence of studies meeting orthomolecular doses means that there is a dearth of direct evidence supporting the effectiveness of orthomolecular interventions in treating parasitic infections. Without studies specifically designed to investigate this question, it is challenging to draw firm conclusions about the efficacy of orthomolecular medicine in this context.
- Uncertainty Regarding Optimal DosagesOrthomolecular medicine emphasizes the use of high-dose nutrient supplementation to restore optimal physiological functioning. However, without studies evaluating the efficacy of orthomolecular doses for parasitic infections, it remains unclear what dosage levels are most effective in combating these diseases. This uncertainty hinders the ability to develop evidence-based treatment guidelines or recommendations in the field of orthomolecular medicine for parasitic infections.
- Potential Overlooked Benefits or RisksStudies investigating orthomolecular interventions at lower doses may still provide valuable insights into their potential benefits or risks in treating parasitic infections. However, by focusing solely on studies meeting orthomolecular doses, the review may overlook relevant findings that could inform clinical practice or future research directions.
- Implications for Future ResearchThe absence of studies meeting orthomolecular doses underscores the need for further research in this area. Future studies should aim to evaluate the efficacy of orthomolecular interventions at doses considered orthomolecular specifically for parasitic infections. This would help fill the existing gap in the literature and provide more robust evidence to guide clinical decision-making.
While the absence of studies meeting orthomolecular doses for parasitic infections limits the ability to draw definitive conclusions, it highlights the need for continued research efforts to explore the potential role of orthomolecular medicine in treating these diseases.
There could be several potential reasons for the absence of studies meeting orthomolecular doses for parasitic infections including but not limited to, lack of research focus, safety concerns, regulatory hurdles, limited funding and the complexity of parasitic infections.
Conclusion
While orthomolecular interventions, particularly vitamin supplementation, have shown promise in other health conditions, their efficacy in treating human parasitic infections remains uncertain due to the lack of direct evidence.
Clinicians may default to conventional antiparasitic drugs, which have established efficacy but may also be associated with limitations such as drug resistance and toxicity. In the absence of evidence supporting orthomolecular interventions, patients may miss out on potentially effective treatments that could complement or enhance conventional therapy.
Future studies should aim to fill this gap in the literature by evaluating the effectiveness of orthomolecular medicine specifically for parasitic infections. Additionally, research efforts should focus on optimizing intervention protocols, exploring potential synergistic effects with conventional therapies, and assessing long-term outcomes and cost-effectiveness.
High-dose nutrient supplementation, while potentially beneficial, may also pose risks of adverse effects, drug interactions, and unintended consequences. Moreover, the use of orthomolecular therapy raises questions regarding patient autonomy, informed consent, and equitable access to treatment. Addressing these ethical considerations is essential to ensure the responsible and ethical implementation of orthomolecular interventions in clinical practice.
Integrating orthomolecular approaches into existing public health strategies may offer a promising avenue for combating human parasitic infections and reducing the global disease burden. In alignment with the One Health approach, the integration of orthomolecular medicine holds potential to address parasitic infections by emphasizing optimal nutrition and the therapeutic use of natural substances, thereby supporting the interconnected health of humans, animals, and ecosystems.
Review Limitations
The primary limitation of this review is the absence of studies meeting orthomolecular doses for parasitic infections, which precludes definitive conclusions regarding the efficacy of orthomolecular interventions in this context. Additionally, the variability in study designs, population characteristics, and outcome measures among the included studies may introduce heterogeneity and limit the generalization of findings.
Publication bias may also have influenced the results, as studies reporting positive outcomes are more likely to be published. Furthermore, the quality of evidence ranged from moderate to low, primarily due to methodological limitations such as inadequate blinding, incomplete outcome data, and risk of bias. Lastly, the potential interactions between orthomolecular interventions and conventional treatments were not systematically assessed, which could impact treatment efficacy and safety.
Disclosure Statement
Some data were unavailable at the time of publication (DUATOP). The conclusions drawn in this manuscript are based on the available data.
The APPENDIX appears below the references.
References
Abbeddou S, Yakes Jimenez E, Somé JW, Ouédraogo JB, Brown KH, Hess SY (2017). Small – quantity lipid-based nutrient supplements containing different amounts of zinc along with diarrhea and malaria treatment increase iron and vitamin A status and reduce anemia prevalence, but do not affect zinc status in young Burkinabe children: a cluster-randomized trial. BMC Pediatr. Feb 2;17(1):46. doi: 10.1186/s12887-016-0765-9. PMID: 28152989; PMCID: PMC5288861.
Aimone AM, Brown P, Owusu-Agyei S, Zlotkin SH, Cole DC (2017). Impact of iron fortification on the geospatial patterns of malaria and non-malaria infection risk among young children: a secondary spatial analysis of clinical trial data from Ghana. BMJ Open. Jun 6;7(5):e013192. doi: 10.1136/bmjopen-2016-013192. PMID: 28592572; PMCID: PMC5734205.
Ajayi AA, Akinleye AO, Udoh SJ, Ajayi OO, Oyelese O, Ijaware CO (1991). The effects of prednisolone and niacin on chloroquine-induced pruritus in malaria. Eur J Clin Pharmacol. 41(4):383-5. doi: 10.1007/BF00314973. PMID: 1804657.
Akenzua GI, Ihongbe JC, Imasuen IW (1985). Haemopoietic response of Nigerian village children to iron, folate supplementation and malaria prophylaxis. J Trop Pediatr. Feb;31(1):59-62. doi: 10.1093/tropej/31.1.59. PMID: 3981699.
Al-Mekhlafi HM, Anuar TS, Al-Zabedi EM, Al-Maktari MT, Mahdy MA, Ahmed A, Sallam AA, Abdullah WA, Moktar N, Surin J (2014). Does vitamin A supplementation protect schoolchildren from acquiring soil-transmitted helminthiasis? A randomized controlled trial. Parasit Vectors. Aug 15;7:367. doi: 10.1186/1756-3305-7-367. PMID: 25127885; PMCID: PMC4141119.
Araújo A, Reinhard K, Ferreira LF, Pucu E, & Chieffi PP (2003). Human intestinal parasites in the past: new findings and a review. Memórias do Instituto Oswaldo Cruz, 98(Suppl 1). https://doi.org/10.1590/S0074-02762003000900016
Arete-Zoe, LLC (2017). Availability of essential medicines in the Czech Republic. Arete- Zoe, LLC.
Artavanis-Tsakonas K, Tongren JE, Riley EM (2003). The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol. Aug;133(2):145-52. doi: 10.1046/j.1365-2249.2003.02174.x. PMID: 12869017; PMCID: PMC1808775.
Ascenzi P, Bocedi A, Gradoni L (2003). The Anti-Parasitic Effects of Nitric Oxide. IUBMB Life, 55(9), 573–578. https://doi.org/10.1080/15216540310001639265
Ash, M (2011). Vitamin A: The Key to Immune Tolerance in the Gut. Journal of Orthomolecular Medicine, 26(1), 7.
Ayoya MA, Spiekermann-Brouwer GM, Traoré AK, Garza C (2012). Effect on school attendance and performance of iron and multiple micronutrients as adjunct to drug treatment of Schistosoma-infected anemic schoolchildren. Food Nutr Bull. Dec; 33(4):235-41. doi: 10.1177/156482651203300403. PMID: 23424889.
Barakat R, Abou El-Ela NE, Sharaf S, El Sagheer O, Selim S, Tallima H, Bruins MJ, Hadley KB, El Ridi R (2015). Efficacy and safety of arachidonic acid for treatment of school-age children in Schistosoma mansoni high-endemicity regions. Am J Trop Med Hyg. Apr;92(4):797-804. doi: 10.4269/ajtmh.14-0675. Epub 2015 Jan 26. PMID: 25624403; PMCID: PMC4385776.
Bektas A, Schurman SH, Sen R, Ferrucci L (2018). Aging, inflammation and the environment. Experimental gerontology, 105, 10-18.
Bogitsh BJ, Carter CE, Oeltmann TN (2013). Human Parasitology (4th ed.). Elsevier Inc.
Brabin L, Roberts SA, Gies S, Nelson A, Diallo S, Stewart CJ, Kazienga A, Birtles J, Ouedraogo S, Claeys Y, Tinto H, d’Alessandro U, Faragher EB, Brabin B (2017). Effects of long- term weekly iron and folic acid supplementation on lower genital tract infection – a double blind, randomised controlled trial in Burkina Faso. BMC Med. Nov 23;15(1):206. doi: 10.1186/s12916-017-0967-5. PMID: 29166928; PMCID: PMC5700548.
Brenner A (1982). The effects of megadoses of selected B complex vitamins on children with hyperkinesis: controlled studies with long-term follow-up. J Learn Disabil. May;15(5):258-64. doi: 10.1177/002221948201500501. PMID: 7086283.
Bresnahan KA, Chileshe J, Tanumihardjo SA (2014). Quantification of food and nutrient intakes in Zambian children with and without malaria under controlled feedingconditions. Experimental Biology and Medicine. 239(1):45-51. doi:10.1177/1535370213510661
Buchmann K (2022). Antiparasitic Immune Responses. In Principles of Fish Immunology (pp. 535–563). Retrieved from Springer.
Carter JP, Grivetti LE, Davis JT, Nasiff S, Mansour A, Mousa WA, Atta Alaa- el-Din Patwardhan VN, Abdel Moneim M, Abdou IA, Darby WJ (1969). Growth and sexual development of adolescent Egyptian village boys: Effects of zinc, iron, and placebo supplementation. The American Journal of Clinical Nutrition, 22(1), 59-78. https://doi.org/10.1093/ajcn/22.1.59.
Carter S (2019). Orthomolecular Medicine. Integr Med (Encinitas). Jun;18(3):74. PMID: 32549818; PMCID: PMC7217400.
Campbell S, Soman-Faulkner K (2024). Antiparasitic Drugs. [Updated 2023 May 29]. In: StatPearls [Internet]. Treasure Island (FL): StatsPearls Publishing
Cathcart R, Cott A, Foster HD (2014). Orthomolecular Treatment of Chronic Disease: 65 Experts on Therapeutic and Preventive Nutrition. Turner Publishing Company.
Chandrasiri UP, Fowkes FJ, Richards JS, Langer C, Fan YM, Taylor SM, Beeson JG, Dewey KG, Maleta K, Ashorn P, Rogerson SJ (2015). The impact of lipid-based nutrient supplementation on anti-malarial antibodies in pregnant women in a randomized controlled trial. Malar J. May 10;14:193. doi: 10.1186/s12936-015-0707-2. PMID: 25957793; PMCID: PMC4438573.
Choi J, Kim DY, Choue R, Lim H (2017). Effects of Vitamin C Supplementation on Plasma and Urinary Vitamin C Concentration in Korean Women. Clin Nutr Res. Jul;6(3):198-205. doi: 10.7762/cnr.2017.6.3.198. Epub 2017 Jul 28. PMID: 28770182; PMCID: PMC5539213.
Parul C, Shahid F, Rizvi A, Klemm RD, Bhutta ZA (2009). Treatment response to standard of care for severe anemia in pregnant women and effect of multivitamins and enhanced anthelminthics. Am J Clin Nutr. Mar;89(3):853-61. doi: 10.3945/ajcn.2008.26826. Epub 2009 Jan 28. PMID: 19176737.
Coop RL, Kyriazakis I (1999). Nutrition–parasite interaction. Veterinary parasitology, 84(3-4), 187-204.
Cox FEG (2002). History of human parasitology. Clinical Microbiology Reviews, 15(4), 595-612. doi:10.1128/CMR.15.4.595-612.2002.
Darling AM, Mugusi FM, Etheredge AJ, Gunaratna NS, Abioye AI, Aboud S, Duggan C, Mongi R, Spiegelman D, Roberts D, Hamer DH, Kain KC, Fawzi WW (2017). Vitamin A and Zinc Supplementation Among Pregnant Women to Prevent Placental Malaria: A Randomized, Double-Blind, Placebo-Controlled Trial in Tanzania. Am J Trop Med Hyg. Apr;96(4):826-834. doi: 10.4269/ajtmh.16-0599. Epub 2017 Jan 23. PMID: 28115667; PMCID: PMC5392628.
De Gier B, Campos Ponce M, van de Bor M, Doak CM, Polman K (2014). Helminth infections and micronutrients in school-age children: a systematic review and meta-analysis. The American Journal of Clinical Nutrition, 99(6), 1499-1509. https://doi.org/10.3945/ajcn.113.069955
Di Giovanni F, Wilke AB, Beier JC, Pombi M, Mendoza-Roldan JA, Desneux N, Benelli G (2021). Parasitic strategies of arthropods of medical and veterinary importance. Entomologia generalis, 41(5).
Dolllimore N, Cutts F, Newton Binka F, Ross DA, Sutkover Morris S (1997). Measles incidence, case fatality, and delayed mortality in children with or without vitamin A supplementation in rural Ghana. American Journal of Epidemiology. 146(8):646‐54.
Dutta S, Goodrich JM, Dolinoy DC, Ruden DM (2023). Biological Aging Acceleration Due to Environmental Exposures: An Exciting New Direction in Toxicogenomics Research. Genes, 15(1), 16.
Egbi G, Ayi I, Saalia FK, et al. (2015). Impact of Cowpea-Based Food Containing Fish Meal Served With Vitamin C–Rich Drink on Iron Stores and Hemoglobin Concentrations in Ghanaian Schoolchildren in a Malaria Endemic Area. Food and Nutrition Bulletin. 36(3):264-
275. doi:10.1177/0379572115596253
Ercumen A, Benjamin-Chung J, Arnold BF, Lin A, Hubbard AE, Stewart C, Rahman Z, Parvez SM, Unicomb L, Rahman M, Haque R, Colford JM Jr, Luby SP (2019). Effects of water, sanitation, handwashing and nutritional interventions on soil-transmitted helminth infections in young children: A cluster-randomized controlled trial in rural Bangladesh. PLoS Negl Trop Dis. May 3;13(5):e0007323. doi: 10.1371/journal.pntd.0007323. PMID: 31050672; PMCID: PMC6519840.
Forman E & Maryanti E (2021). What Makes the Elderly Prone to Parasitic Infection? Asian Journal of Research in Infectious Diseases, 6(1), 24-31. https://doi.org/10.9734/AJRID/2021/v6i130183
Gara SN, Madaki AJ, Thacher TD (2010). A comparison of iron and folate with folate alone in hematologic recovery of children treated for acute malaria. Am J Trop Med Hyg.Oct;83(4):843-7. doi: 10.4269/ajtmh.2010.10-0170. PMID: 20889877; PMCID: PMC2946754.
Gibson RS, Yeudall F, Drost N, Mtitimuni BM, Cullinan TR (2003). Experiences of a community-based dietary intervention to enhance micronutrient adequacy of diets low in animal source foods and high in phytate: a case study in rural Malawian children. J Nutr. Nov;133(11 Suppl 2):3992S-3999S. doi: 10.1093/jn/133.11.3992S. PMID: 14672301.
Gies S, Diallo S, Roberts SA, Kazienga A, Powney M, Brabin L, Ouedraogo S, Swinkels DW, Geurts-Moespot AJ, Claeys Y, D’Alessandro U, Tinto H, Faragher B, Brabin B (2018). Effects of Weekly Iron and Folic Acid Supplements on Malaria Risk in Nulliparous Women in Burkina Faso: A Periconceptional, Double-Blind, Randomized Controlled Noninferiority Trial. J Infect Dis. Aug 24;218(7):1099-1109. doi: 10.1093/infdis/jiy257. PMID: 29733403; PMCID: PMC6107738.
Gies S, Roberts SA, Diallo S, Lompo OM, Tinto H, Brabin BJ (2021). Risk of malaria in young children after periconceptional iron supplementation. Matern Child Nutr. Apr;17(2):e13106. doi: 10.1111/mcn.13106. Epub 2020 Nov 25. PMID: 33236840; PMCID: PMC7988873.
Glinz D, Hurrell RF, Righetti AA, Zeder C, Adiossan LG, Tjalsma H, Utzinger J, Zimmermann MB, N’Goran EK, Wegmüller R (2015). In Ivorian school-age children, infection with hookworm does not reduce dietary iron absorption or systemic iron utilization, whereas afebrile Plasmodium falciparum infection reduces iron absorption by half. Am J Clin Nutr. Mar;101(3):462-70. doi: 10.3945/ajcn.114.090175. Epub 2015 Feb 4. PMID: 25733630.
Goodwin JS, Garry PJ (1983). Relationship between megadose vitamin supplementation and immunological function in a healthy elderly population. Clin Exp Immunol. Mar;51(3):647-53. PMID: 6851251; PMCID: PMC1536802.
Grazioso CF, Isalgué M, de Ramírez I, Ruz M, Solomons NW (1993). The effect of zinc supplementation on parasitic reinfestation of Guatemalan schoolchildren. Am J Clin Nutr. May;57(5):673-8. doi: 10.1093/ajcn/57.5.673. PMID: 8480685.
Guzman-Rivero M, Verduguez-Orellana A, Montaño K, Cloetens L, Rojas E, Åkesson B, Sejas E (2015). The immune response in patients with cutaneous leishmaniasis and the influence of zinc supplementation. Biomed Pharmacother. Feb;69:56-62. doi: 10.1016/j.biopha.2014.11.006. Epub 2014 Nov 15. PMID: 25661338.
Hemat RAS (2004). Principles of orthomolecularism. Urotext.
Hoang BX, Shaw G, Fang W, Han B (2020). Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. Dec;23:256-262. doi: 10.1016/j.jgar.2020.09.025. Epub 2020 Oct 13. PMID: 33065330; PMCID: PMC7553131. hoa
Hoeksema M, van Eijk M, Haagsman HP, Hartshorn KL (2016). Histones as mediators of host defense, inflammation and thrombosis. Future Microbiology, 11(3). https://doi.org/10.2217/fmb.15.151
Hoffer A (1976). Megavitamin Therapy for Different Cases. Orthomolecular Psychiatry, 5(3), 169-182.
Hoffer A (1983). Criticism. Journal of Orthomolecular Medicine, 12(4).
Holick MF (2002). Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Current Opinion in Endocrinology & Diabetes 9(1):p 87-98, February.
Holick MF (2003). Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. The American Journal of Clinical Nutrition, 78(suppl), 6S- 10S.
Huemer R (2006). Chronic Renal Disease: Orthomolecular Ramifications. Journal of Orthomolecular Medicine, 21(1).
Imam TS (2009). The complexities in the classification of protozoa: a challenge to parasitologists. Bayero Journal of Pure and Applied Sciences, 2(2), 159-164.
Klenner FR (1954). The treatment of trichinosis with massive doses of vitamin C and para-aminobenzoic acid. Tri-State Medical Journal, 2(2), 25–30.
Król G, Tomaszewska A, Wróbel G, Paprocka P, Durnaś B, Piktel E, Bucki R (2019). Toxicity of parasites and their unconventional use in medicine. Ann Agric Environ Med., 26(4), 523-531. https://doi.org/10.26444/aaem/109665
Kucik CJ, Martin GL, Sortor BV (2004). Common Intestinal Parasites. American Family Physician, 69(5), 1161-1169.
Kotepui M, Wilairatana P, Mala W, Kotepui KU, Masangkay FR, Wangdi K (2023). Effects of Daily Zinc Alone or in Combination with Other Nutrient Supplements on the Risk of Malaria Parasitaemia: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. Jun 23;15(13):2855. doi: 10.3390/nu15132855. PMID: 37447182; PMCID: PMC10346149.
Long KZ, Rosado JL, Montoya Y, de Lourdes Solano M, Hertzmark E, DuPont HL, Santos JI (2007). Effect of vitamin A and zinc supplementation on gastrointestinal parasitic infections among Mexican children. Pediatrics. Oct;120(4):e846-55. doi: 10.1542/peds.2006- 2187. PMID: 17908741.
Leenstra T, Kariuki SK, Kurtis JD, Oloo AJ, Kager PA, ter Kuile FO (2009). The effect of weekly iron and vitamin A supplementation on hemoglobin levels and iron status in adolescent schoolgirls in western Kenya. Eur J Clin Nutr. Feb;63(2):173-82. doi: 10.1038/sj.ejcn.1602919. Epub 2007 Oct 10. PMID: 17928808.
Layden AJ, Täse K, Finkelstein JL (2018). Neglected tropical diseases and vitamin B12: a review of the current evidence. Trans R Soc Trop Med Hyg. Oct 1;112(10):423-435. doi: 10.1093/trstmh/try078. Erratum in: Trans R Soc Trop Med Hyg. 2019 May 1;113(5):292. PMID: 30165408; PMCID: PMC6457089.
Magalhães LS, Melo EV, Damascena NP, Albuquerque ACB, Santos CNO, Rebouças MC, Bezerra MO, Louzada da Silva R, de Oliveira FA, Santos PL, da Silva JS, Lipscomb MW, da Silva ÂM, de Jesus AR, de Almeida RP (2022). Use of N-acetylcysteine as treatment adjuvant regulates immune response in visceral leishmaniasis: Pilot clinical trial and in
vitro experiments. Front Cell Infect Microbiol. Nov 24;12:1045668. doi: 10.3389/fcimb.2022.1045668. PMID: 36506010; PMCID: PMC9730326.
Malmberg KJ, Lenkei R, Petersson M, Ohlum T, Ichihara F, Glimelius B, Frodin JE (2002). A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clinical Cancer Research, 8(6), 1772-8.
Mayxay M, Khanthavong M, Cox L, Sichanthongthip O, Imwong M, Pongvongsa T, Hongvanthong B, Phompida S, Vanisaveth V, White NJ, Newton PN (2014). Thiamin supplementation does not reduce the frequency of adverse events after anti-malarial therapy among patients with falciparum malaria in southern Laos. Malar J. Jul 15;13:275. doi: 10.1186/1475-2875-13-275. PMID: 25027701; PMCID: PMC4105794.
Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, Greenwood B, Walraven G (2006). Lack of inhibition of the anti-malarial action of sulfadoxine-pyrimethamine by folic acid supplementation when used for intermittent preventive treatment in Gambian primigravidae. Am J Trop Med Hyg. Jun;74(6):960-4. PMID: 16760504.
McDonald CM, Manji KP, Kisenge R, Aboud S, Spiegelman D, Fawzi WW, Duggan CP (2015). Daily Zinc but Not Multivitamin Supplementation Reduces Diarrhea and Upper Respiratory Infections in Tanzanian Infants: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Nutr. 2015 Sep;145(9):2153-60. doi: 10.3945/jn.115.212308. Epub Jul 22. PMID: 26203094; PMCID: PMC4548161.
McVeigh P (2020). Post-genomic progress in helminth parasitology. Parasitology. 147(8):835- 840. doi:10.1017/S0031182020000591
Migliore L, Coppedè F (2009). Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 674(1-2), 73-84.
Mikirova N (2020). Continuous ascorbate infusions in the management of patients with advanced colon cancer. Journal of Orthomolecular Medicine, 35(2).
Muhumuza S, Olsen A, Katahoire A, Kiragga AN, Nuwaha F (2014). Effectiveness of a pre- treatment snack on the uptake of mass treatment for schistosomiasis in Uganda: a cluster randomized trial. PLoS Med. May 13;11(5):e1001640. doi: 10.1371/journal.pmed.1001640. PMID: 24824051; PMCID: PMC4019501.
Murphy MSQ, Muldoon KA, Sheyholislami H, Behan N, Lamers Y, Rybak N, White RR, Harvey ALJ, Gaudet LM, Smith GN, Walker MC, Wen SW, MacFarlane AJ (2021). Impact of high- dose folic acid supplementation in pregnancy on biomarkers of folate status and 1-carbon metabolism: An ancillary study of the Folic Acid Clinical Trial (FACT). Am J Clin Nutr. May 8;113(5):1361-1371. doi: 10.1093/ajcn/nqaa407. PMID: 33675351; PMCID: PMC8106758.
Mutuku MW (2020). Compatibility of Schistosoma Mansoni and Its Intermediate Host Snails (Biomphalaria Spp) in Relation to Transmission of Intestinal Schistosomiasis in Kenya (Doctoral dissertation, University of Nairobi).
Müller O, Becher H, van Zweeden AB, Ye Y, Diallo DA, Konate AT, Gbangou A, Kouyate B, Garenne M (2001). Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomised double blind placebo controlled trial. BMJ.Jun 30;322(7302):1567. doi: 10.1136/bmj.322.7302.1567. PMID: 11431296; PMCID: PMC33513.
Mwanakasale V, Siziya S, Mwansa J, Koukounari A, Fenwick A (2009). Impact of iron supplementation on schistosomiasis control in Zambian school children in a highly endemic area. Malawi Med J. Mar;21(1):12-8. doi: 10.4314/mmj.v21i1.10982. PMID: 19780472; PMCID: PMC3345721.
Nag VL, Kalita JM (2022). Epidemiology of parasitic infections. In Textbook of Parasitic Zoonoses (pp. 1-14). Microbial Zoonoses.
Nur E, Brandjes DP, Teerlink T, Otten HM, Oude Elferink RP, Muskiet F, Evers LM, ten Cate H, Biemond BJ, Duits AJ, Schnog JJ, CURAMA study group (2012). N-acetylcysteine reduces oxidative stress in sickle cell patients. Ann Hematol. Jul;91(7):1097-105. doi: 10.1007/s00277-011-1404-z. Epub 2012 Feb 10. PMID: 22318468; PMCID: PMC3368118.
Nzila A, Okombo J, Hyde J (2016). Malaria in the Era of Food Fortification With Folic Acid. Food and Nutrition Bulletin. ;37(2):153-163. doi:10.1177/0379572116634511
Olney DK, Kariger PK, Stoltzfus RJ, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Pollitt E (2013). Developmental effects of micronutrient supplementation and malaria in Zanzibari children. Early Hum Dev. Sep;89(9):667-74. doi: 10.1016/j.earlhumdev.2013.04.013. Epub 2013 May 28. PMID: 23725789.
Olsen, J. Nawiri, H. Friis,(2000). The impact of iron supplementation on reinfection with intestinal helminths and Schistosoma mansoni in western Kenya,Transactions of the Royal Society of Tropical Medicine and Hygiene,Volume 94, Issue 5, Pages 493-499, ISSN 0035-9203, https://doi.org/10.1016/S0035-9203(00)90063-4.
Overbeck S, Rink L, Haase H (2008). Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Archives of Immunology and Therapy Experimental, 56, 15–30. DOI: 10.1007/s00005-008-0003-8
Owusu-Agyei S, Newton S, Mahama E, Febir LG, Ali M, Adjei K, Tchum K, Alhassan L, Moleah T, Tanumihardjo SA (2013). Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr J. Sep 23;12:131. doi: 10.1186/1475-2891-12-131. PMID: 24330422; PMCID: PMC3850154.
Papier K, Williams GM, Luceres-Catubig R, Ahmed F, Olveda RM, McManus DP, Chy D, Chau TNP, Gray DJ, Ross AGP (2014). Childhood malnutrition and parasitic helminth interactions. Clinical Infectious Diseases, 59(2), 234–243. https://doi.org/10.1093/cid/ciu211
Pataracchia RJ (2010). Orthomolecular treatment response. Orthomolecular Medicine News Service, 25(1).
Pauling L (1968). Orthomolecular psychiatry. Varying the concentrations of substances normally present in the human body may control mental disease. Science. Apr 19;160(3825):265-71. doi: 10.1126/science.160.3825.265. PMID: 5641253.
Pink R, Hudson A, Mouriès MA, Bendig M (2005). Opportunities and challenges in antiparasitic drug discovery. Nature review Drug discovery, 4(9), 727-740.
Prietl B, Treiber G, Pieber TR, Amrein K (2013). Vitamin D and immune function. Nutrients, 5(7), 2502-2521. https://doi.org/10.3390/nu5072502
Prousky J (2013). Orthomolecular Psychiatric Treatments Are Preferable to Mainstream Psychiatric Drugs: A Rational Analysis. Journal of Orthomolecular Medicine, 28(1), 17-32.
Puente V, Demaria A, Frank FM, Batlle A, Lombardo ME (2018). Anti-parasitic effect of vitamin C alone and in combination with benznidazole against Trypanosoma cruzi. PLoS Neglected Tropical Diseases, 12(9), e0006764. https://doi.org/10.1371/journal.pntd.0006764
Ramos-Martínez E, Gutierrez-Kobeh L, Villaseñor-Cardoso MI (2015). The role of vitamin D in the control of Leishmania infection. Canadian Journal of Physiology and Pharmacology, 93(5), 373-377. https://doi.org/10.1139/cjpp-2014-0372
Ranasinghe S, Aspinall S, Beynon A, Ash A, Lymbery A (2023). Traditional medicinal plants in the treatment of gastrointestinal parasites in humans: A systematic review and meta-analysis of clinical and experimental evidence. Phytotherapy Research. 37:3675–3687
Roberts LS (2004). Foundations of Parasitology (7th ed.). McGraw-Hill
Rosado JL, Caamaño MC, Montoya YA, de Lourdes Solano M, Santos JI, Long KZ (2009). Interaction of zinc or vitamin A supplementation and specific parasite infections on Mexican infants’ growth: a randomized clinical trial. Eur J Clin Nutr. 2009 Oct;63(10):1176-84. doi: 10.1038/ejcn.2009.53. Epub Jul 22. PMID: 19623197.
Rosenblatt JE (1992). Antiparasitic agents. In Mayo Clinic Proceedings (Vol. 67, No. 3, pp.276-287). Elsevier.
Saaka M, Oosthuizen J, Beatty S (2009). Effect of joint iron and zinc supplementation on malarial infection and anaemia. East Afr J Public Health. Apr;6(1): 55-62. doi: 10.4314/eajph.v6i1.45748. PMID: 20000066.
Saul AW (2003). Vitamin E: A Cure in Search of Recognition. Journal of Orthomolecular Medicine, 18 (3-4).
Scott ME, Koski KG (2000). Zinc deficiency impairs immune responses against parasitic nematode infections at intestinal and systemic sites. The Journal of Nutrition, 130(5S Suppl), 1412S-20S. DOI: 10.1093/jn/130.5.1412S
Semba RD (2007). Vitamin A and immunity to viral, bacterial and protozoan infections. Advances in Experimental Medicine and Biology, 587, 19-34. DOI: 10.1007/978-1-4020- 5133-3_2
Selim S, El Sagheer O, El Amir A, Barakat R, Hadley K, Bruins MJ, El Ridi R (2014). Efficacy and safety of arachidonic acid for treatment of Schistosoma mansoni-infected children in Menoufiya, Egypt. Am J Trop Med Hyg. 2014 Nov;91(5):973-81. doi: 10.4269/ajtmh.14- 0328. Epub Sep 22. PMID: 25246692; PMCID: PMC4228895.
Seshadri S, Hirode K, Naik P, Shah A, Gupta N (1984). An effective intervention to reduce the prevalence of anaemia in children. Indian Journal of Medical Research, 80, 164-173. PMID: 6511008
Shahriar AA, Alpern JD (2020). Antiparasitic drugs in the United States—two roads to high prices. Frontiers in Sociology, 5, 540478.
Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, Alpers MP, West KP (1999). Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. The Lancet, 354(9174), 203-209. https://doi.org/10.1016/S0140-6736(98)08293-2
Shield JM, Vaterlaws AL, Kimber RJ, Payne R, Casey GJ, Blunden RW, Kutkaite D (1981). The relationship of hookworm infection, anaemia and iron status in a Papua New Guinea highland population and the response to treatment with iron and mebendazole. P N G Med J. Mar;24(1):19-34. PMID: 6945770.
Siekmann JH, Allen LH, Bwibo NO, Demment MW, Murphy SP, Neumann CG (2003). Kenyan school children have multiple micronutrient deficiencies, but increased plasma vitamin B-12 is the only detectable micronutrient response to meat or milk supplementation. J Nutr. Nov;133(11 Suppl 2):3972S-3980S. doi: 10.1093/jn/133.11.3972S. PMID: 14672298.
Snyman JR, de Sommers K, Steinmann MA, Lizamore DJ. Effects of calcitriol on eosinophil activity and antibody responses in patients with schistosomiasis (1997). Eur J Clin Pharmacol. ;52(4):277-80. doi: 10.1007/s002280050289. PMID: 9248764.
Sondo P, et al. (2023). Enhanced effect of seasonal malaria chemoprevention when coupled with nutrients supplementation for preventing malaria in children under 5 years old in Burkina Faso: a randomized open label trial. Malar J. Oct 18;22(1):315. doi: 10.1186/s12936-023-04745-6. PMID: 37853408; PMCID: PMC10585892.
Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB (2020). Oral iron supplementation in iron-deficient women: How much and how often? Molecular Aspects of Medicine, 75, Article 100865.
Suchdev PS, Addo OY, Martorell R, Grant FK, Ruth LJ, Patel MK, Juliao PC, Quick R, Flores-Ayala R (2016). Effects of community-based sales of micronutrient powders on morbidity episodes in preschool children in Western Kenya. Am J Clin Nutr. Mar;103(3):934-41. doi: 10.3945/ajcn.115.118000. Epub 2016 Feb 10. PMID: 26864367; PMCID: PMC4845747.
Tomashek KM, Woodruff BA, Gotway CA, Bloland P, Mbaruku G (2001). Randomized intervention study comparing several regimens for the treatment of moderate anemia among refugee children in Kigoma Region, Tanzania. Am J Trop Med Hyg. Mar-Apr;64(3- 4):164-71. doi: 10.4269/ajtmh.2001.64.164. PMID: 11442213.
Tallima H, Hanna VS, El Ridi R (2020). Arachidonic Acid Is a Safe and Efficacious Schistosomicide, and an Endoschistosomicide in Natural and Experimental Infections, and Cysteine Peptidase Vaccinated Hosts. Frontiers in Immunology, 11. https://doi.org/10.3389/fimmu.2020.609994
Toole JF (2002). Vitamin intervention for stroke prevention. J Neurol Sci. Nov 15;203- 204:121-4. doi: 10.1016/s0022-510x(02)00265-4. PMID: 12417369.
Ung L, Stothard JR, Phalkey R, Azman AS, Chodosh J, Hanage WP, Standley CJ (2021). Towards global control of parasitic diseases in the Covid-19 era: One Health and the future of multisectoral global health governance. Advances in Parasitology, 114, 1-26. https://doi.org/10.1016/bs.apar.2021.08.007
Villamor E, Fataki MR, Mbise RL, Fawzi WW (2003). Malaria parasitaemia in relation to HIV status and vitamin A supplementation among pre-school children. Trop Med Int Health. Dec; 8(12):1051-61. doi: 10.1046/j.1360-2276.2003.01134.x. PMID: 14641839.
van Hensbroek MB, Morris-Jones S, Meisner S, Jaffar S, Bayo L, Dackour R, Phillips C, Greenwood BM (1995). Iron, but not folic acid, combined with effective antimalarial therapy promotes haematological recovery in African children after acute falciparum malaria. Trans R Soc Trop Med Hyg. Nov-Dec;89(6):672-6. doi: 10.1016/0035- 9203(95)90438-7. PMID: 8594693.
Watt JYC (1944). The Influence of Vitamins B1 (thiamin) and B2 (Riboflavin) upon the Resistance of Rats to Infection with Nippostrongylus muris. American Journal of Hygiene, 39(2), 145-151.
Zeba AN, Sorgho H, Rouamba N, Zongo I, Rouamba J, Guiguemdé RT, Hamer DH, Mokhtar N, Ouedraogo JB (2008). Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: a randomized double blind trial. Nutr J. Jan 31;7:7. doi: 10.1186/1475-2891-7-7. PMID: 18237394; PMCID: PMC2254644.
Zughaier SM, Lubberts E, Bener A (2020). Editorial: Immune-Modulatory Effects of Vitamin D. Frontiers in Immunology, 11. https://doi.org/10.3389/fimmu.2020.596611
Appendix
Selected studies and data utilized.
1. Iron Studies
2. Vitamin A Studies
3. Multivitamin Studies

4. Zinc Studies
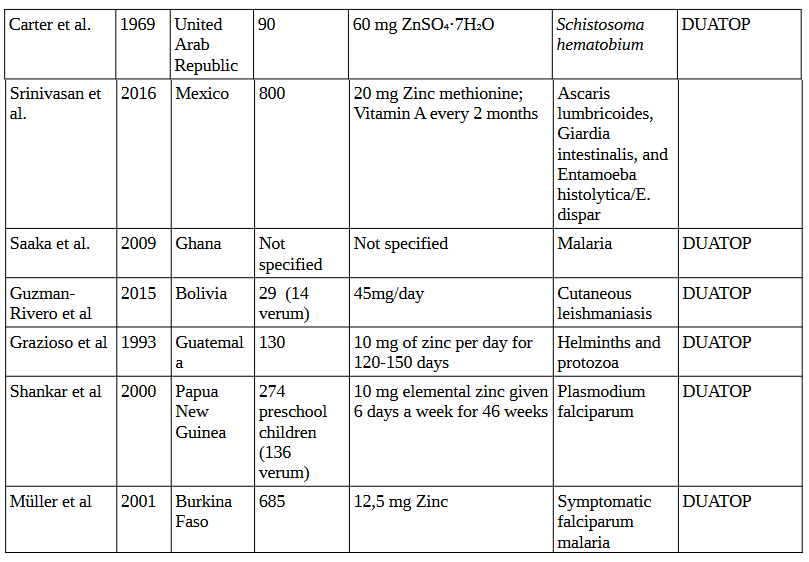
5. Vitamin B1 (Thiamine) Studies

6. Vitamin B3 (Niacin) Studies
![]()
7. Vitamin B12 (Cobalamin) Studies

8. Folate Studies

9. Vitamin C Studies

10. Vitamin D Studies

11. N-acetylcysteine (NAC) Studies