Background
First described in 1998, Paediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal infections (PANDAS) is a specific case of Paediatric Acute-onset Neuropsychiatric Syndrome (PANS) (Quagliarello et al., 2018). PANS is a ‘later, broader iteration of PANDAS’ (Wilbur et al., 2019, p. 85), that englobes various neuropsychiatric conditions that are triggered suddenly. The conditions are thought to be caused by infections, metabolic issues, and/or environmental factors (Swedo et al., 1998; Wilbur et al., 2019). Given the temporal association between the onset of symptoms and the group A streptococcal infection (GAS), it is often hypothesised that this range of symptoms is caused by an autoimmune response to the GAS infection (Sigra, Hesselmark, & Bejerot, 2018).
PANDAS symptoms include obsessive and compulsive disorder (OCD), hypersensitivities, depression, insomnia, food restriction, hyperactivity, inattentiveness and in some cases, hallucinations. (Frankovich et al., 2015; Mangiola et al., 2016; Thienemann et al., 2017)
Due to the heterogeneity of its clinical picture, the PANDAS diagnosis is considered controversial, and the understanding of its aetiology remains elusive (Dale et al., 2006; Gamucci et al., 2019; Leckman et al., 2011; Orefici, Cardona, Cox, and Cunningham, 2016).
Similarly, treatment for PANDAS is a complex issue, as the symptoms encompass different areas of psychopathology. Various possible treatments that can be combined differently depending on the patient’s profile: antibiotics, immunomodulatory therapy, tonsillectomy, cognitive and behavioral therapy, non-steroidal anti-inflammatory drugs, antidepressants, antipsychotics, and mood stabilizers (Thienemann et al., 2017). Despite some authors advocating the intake of antibiotics as first-line treatment (Calaprice, Tona, & Murphy, 2018), other studies remain skeptical as to their efficacy, tolerability, and long-term adequacy (Garvey et al., 1999; Sigra et al., 2018). Indeed, Murphy et al. (2017) suggested that antibiotics only alleviate OCD-related symptoms. Similarly, while antibiotics can be of assistance when a GAS infection is in action, there is no evidence supporting the effectiveness of antibiotic courses in preventing exacerbations of PANDAS symptoms (Wilbur et al., 2019). This supports findings of Quagliarello et al. (2018) who state that PANDAS’ inflammatory condition ‘is likely maintained in patients, even after the infection itself has resolved’ (p.12).
In most cases, medications treat symptoms, not the underlying cause. Here, the question to ask is: where is the inflammation coming from and why does it persist? A comprehensive, case-by-case approach is vital, and should predominate when exploring PANDAS and its treatments (Sigra et al., 2018).
Gut Health in Patients With PANDAS
Most research highlights the absence of biological markers for PANDAS (Gamucci et al., 2019). Yet, Quagliariello et al. (2018) have investigated the gut microbiota composition in a sample of 30 children suffering from PANDAS. The results suggest a relationship between PANDAS and gut flora composition. Younger participants displayed all the signs of gut dysbiosis. Their gut flora were less biodiverse than those of their control peers.
PANDAS participants displayed lower levels of clostridia and roseburia, bacteria that are critical in defending our bodies against external infections (Lopetuso, Scaldaferri, Petito, & Gasbarrini, 2013) and preserving both gut homeostasis and the gut barrier (Velasquez-Manoff, 2015). Finally, erysipelotrichaceae bacteria were missing from the microbiota of PANDAS participants. Interestingly, findings from Palm et al. (2014) and Kawikova et al. (2010) suggest a correlation between the absence of erysipelotrichaceae and immunologic disorders.
The above findings are crucial as they suggest not only that there might be biomarkers for PANDAS, but that these lie in the gut. This resonates with a growing concept in today’s literature: the gut-brain axis.
Contrary to our genome, microbiota are flexible and responsive to environmental changes (Lloyd-Price et al., 2017). This makes our microbial inhabitants a potential target for treatment (Cryan et al., 2019). Concerning PANDAS, research has already demonstrated the validity of such an approach. For instance, in Calaprice, Tona, and Murphy’s (2018) large sample, 352 families stated that alternative medicine treatments with probiotics, Omega 3, vitamin D, homeopathy and gluten-free diet improved their child’s health state.
GAPS Nutritional Protocol: The Diet That Focuses On The Gut-Brain Axis
In the last two decades, Dr Campbell-McBride has developed a specific protocol that focusses on repairing the gut lining and restoring normal balance in the gut microbiome.
GAPS stands for Gut And Psychology/Physiology Syndrome. Its core principles rely on the need for a healthy microbiome for our body to function correctly.
The Full GAPS Diet eliminates all processed foods, sugar, vegetable oils, grains, starchy vegetables and legumes, and focusses on nutrient-dense foods such as healthy fats and stocks. The GAPS Introduction Diet is designed for deeper healing: it is a stage-by-stage introduction of nourishing foods, starting from the easiest-to-digest to more difficult-to-digest foods, slowly working up to The Full GAPS Diet. As it is crucial to replace the pathogens in our gut with beneficial microbes, probiotics are central to the protocol, both from fermented foods and commercial preparations (please see our companion article GAPS Nutritional Protocol: how healing the gut removes the basis for all chronic diseases, for a thorough description of the GAPS Nutritional Protocol).
Although the foundations of such a protocol have been accepted by the medical community for a long time (Campbell-McBride, 2020; Gottschall, 1994; Haas, 1951; Price, 2016), the scientific literature remains anecdotal. Throughout this case report, the current study aims to shed light on the validity of the GAPS Nutritional Protocol and highlight why this approach is fundamental for the treatment of PANDAS.
Case Presentation
Medical and Developmental History
George was born in 2000 through a C-section. He was breastfed for six months, after which he was weaned with homemade soups and, on rarer occasions, commercial homogenised foods.
As a child, George displayed signs of insecure attachment. He would follow both his parents all the time, and they struggled to leave him with other people or even on his own. His most common words were “mom don’t leave me”. Bedtime was complicated as George appeared to suffer from nightmares and night terrors. He also displayed disturbing digestive issues such as bloating, abdominal pain, and constipation. Mealtimes became problematic, as George was incredibly fussy with food.
At 12 years of age, his insecurities and anxieties became uncontrollable. He suddenly refused to go to school and was clearly overwhelmed by simple tasks. Tics emerged and he would make, as his mother described, “snake-resembling” movements.
For a year and a half, George had to drop out of school. He saw a therapist to whom he confessed all his emerging phobias and OCD-related symptoms. George was full of invasive negative thoughts that conditioned his daily life.
At 14 years of age, after years of questioning and researching, George’s parents had concluded that their son might suffer from a disease called PANDAS. They went to another specialist, who confirmed this diagnosis. In addition, they also diagnosed George with an anxiety disorder. Antibiotic therapy was prescribed in combination with mood stabilizers.
The prescribed medication led to some improvements in eye contact and tics. However, this treatment did not have an impact on George’s anxiety, aggressiveness, and reduced attention span.
When George was 15, his parents decided, in one last attempt, to try a nutritional intervention. They contacted Shantih Coro, a Registered Dietician, and Certified GAPS Practitioner.
Intervention
Before Intervention
George’s diet prior to visiting Mr Coro consisted of a traditional Italian menu. George was a fussy eater; the only things that he ate gladly were sweets and processed carbohydrates. He refused vegetables, fruit, and animal products.
During Intervention
Mr Coro recommended that George start immediately from the GAPS Introduction Diet. It was challenging for an Italian family to live without pasta, bread, pizza, and sugar, so during the first weeks George only removed some of these foods. Yet, his sleep and bowel movements significantly improved. This notable progress encouraged the family to persist with the protocol. For 30 days, George thoroughly followed the GAPS Introduction Diet, after which, all of his symptoms had improved, including the tics. This impressed the whole family deeply and inspired them to persevere with this approach. For six months, George went through the stages of The GAPS Introduction Diet, before transitioning to The Full GAPS Diet. After 18 months of following The GAPS Nutritional Protocol, the PANDAS diagnosis was removed, and all medication was stopped.
Post-Intervention
Qualitative Observations: By following the GAPS Nutritional Protocol, George gradually saw all his symptoms disappear, to the point where the diagnosis was removed, and all medications were stopped. He went back to having a normal social and academic life, after years of communication and learning difficulties linked to his severe anxiety. He demanded, of his own initiative, to go back to public school (public school is harder in Italy, and the fact that George decided to go back by himself was another proof of his recovery). Nowadays, George does not follow the GAPS Diet, but he maintains healthy eating habits and lifestyle. He studies medicine with excellent grades.
Quantitative Analysis: George’s healing following the GAPS Diet is also visible in objective, quantitative data (see Table 1).
TABLE 1. Critical blood test values throughout the GAPS Diet implementation
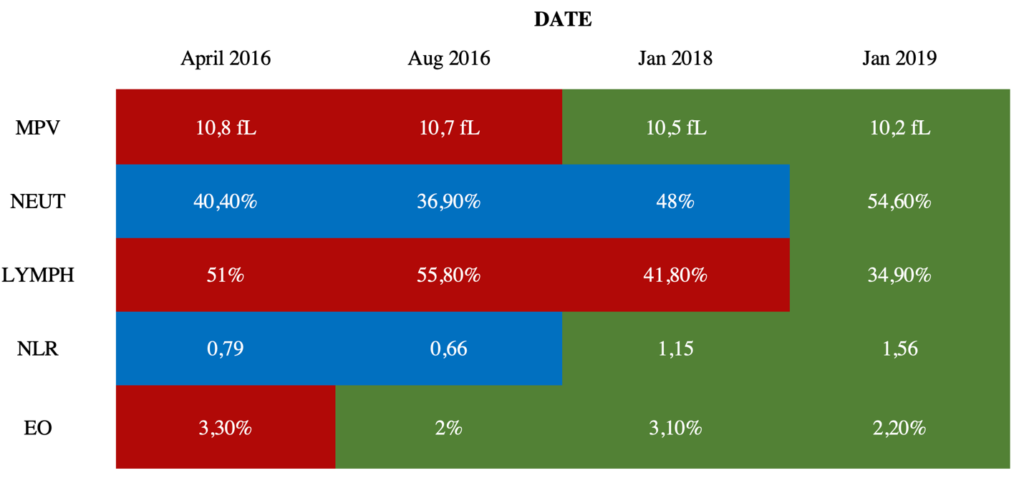
Colour chart: Red cells indicate a significantly high value compared to the norm for the relevant age range; Blue cells indicate a significantly low value compared to the norm for the relevant age range; Green cells indicate a value within normal range.
Mean Platelet Volume (MPV): High MPV has been reported multiple times in cases of sepsis (Ates et al., 2015; Aydemir, Piskin, Akduman, Kokturk, & Aktas, 2015; Shaaban & Safwat, 2020) and has been explored as a biomarker for inflammatory and autoimmune diseases (Schmoeller et al., 2017; Soydinc et al., 2014; Yavuz & Ece, 2014; Yuri Gasparyan, Ayvazyan, Mikhailidis, & Kitas, 2011). In 2016, George’s MPV was significantly high, which indicated that this patient had systemic infection, inflammation, and autoimmunity. Yet, the diagnosis of PANDAS was established in 2012, followed by intensive antibiotic treatments aimed at clearing any possible GAS infection. The child continued being ill until 2018, when, after the GAPS intervention, his MPV went back to normal, and the clinical picture showed disappearance of all symptoms of PANDAS.
Neutrophils (NEUT) and Lymphocytes (LYMPH): NEUT and LYMPH are central in the prevention of autoimmune diseases (Seeley et al., 2008). George’s blood results revealed a significantly low percentage of NEUT in his bloodstream for over two years: this is called chronic neutropenia, which can be indicative of a suppressed autoimmune system and/or an autoimmune disorder (Akhtari, Curtis, & Waller, 2009; Boxer, Greenberg, Boxer, & Stossel, 1975; Boxer, 2012; Bruin et al., 1999; Kolaczkowska & Kubes, 2013; Starkebaum, 2002). After the GAPS intervention, George’s NEUT count had returned to normal (see Table 1). George also displayed significantly high levels of lymphocytes, called lymphocytosis. Lymphocytosis is thought to be caused by bacterial, viral, or parasitic infections, cancers, and autoimmune disorders that lead to chronic inflammation (Hamad & Mangla, 2019). George’s LYMPH percentage was out of the normal range and reflected an inflammatory state. After the GAPS Nutritional Protocol, his LYMPH count was restored to normal (see Table 1).
Neutrophil-to-Lymphocyte Ratio (NLR): George’s blood results depicted a peculiar profile, one of a particularly low neutrophil-to-lymphocyte ratio (NLR) (Forget et al., 2017). Low NLR is associated with chronic inflammation, infection, and autoimmune conditions (Castoldi, Liso, & Aversa, 2014; Dursun, Ozsoylu, & Akyildiz, 2018) and has also been explored as a biomarker for OCD (a prevalent symptom in PANDAS). Uzun et al. (2020) have found an abnormally low NLR ranging between 0.89 and 1.87 in children and adolescents with OCD. In 2016, George’s NLR at 0.66 was lower than the abovementioned range. Doctors had warned George’s family that it might be impossible to revert this. Yet, post-intervention, George’s NLR went completely back to normal (see Table 1).
Eosinophils (EO): A high EO count (eosinophilia) reflects autoimmune issues (Diny, Rose, & Čiháková, 2017; Kovalszki & Weller, 2016; Nagata, Nakagome & Soma, 2020; Wang, Lu, Guo, & Qian, 2009), severe inflammation and/or parasitic infections. Just like his other blood levels, his EO count decreased to a normal range after the GAPS intervention (see Table 1).
Discussion
Key Findings
This case study aimed to explore the GAPS Nutritional Protocol as a treatment for PANS/PANDAS. Both qualitative and quantitative data indicated that George’s condition improved following the GAPS Diet, conceived to heal and seal the ‘leaky’ gut lining and repopulate the gut flora with beneficial microbes.
After 18 months, George saw all his PANDAS-related symptoms disappear – tics, anxiety, hypersensitivity to noise and odours, sleep issues, concentration issues, fine motor issues, digestive issues, restricted eating habits, and quality of eye-contact – to the point where his diagnosis was officially removed.
In addition, the abnormal blood test results that were indicative of the PANDAS condition – EO, NEUT, LYMPH, NLR, MPV – were restored to normal ranges after the GAPS intervention.
Interpretations
Quagliarello et al. (2018) have claimed that PANDAS patients suffer from gut dysbiosis. The current study corroborates these findings and suggests that gut health is central to the treatment of PANDAS.
How did the GAPS Nutritional protocol help George heal from PANDAS?
When George followed the GAPS Nutritional Protocol, his family focussed on removing toxicity from his environment and his food. Removing all sugar, beans, grains, starch, and processed foods re-balanced George’s gut microbiome. Homemade fermented dairy and fermented vegetables helped strengthen his beneficial flora. Food began to be correctly digested and absorbed. In addition, removal of fibre for a period of time (nuts, beans, fruit and raw vegetables) allowed his gut lining to heal. Nourishment from meat stocks and nutrient-dense animal foods – most importantly, collagen – sealed his ‘leaky’ gut. Healthy animal fats and cholesterol-rich foods provided him with the necessary building materials to reinforce every cell in his body and nourish his brain.
Where did George’s gut dysbiosis come from?
Gut dysbiosis is inherited from our parents. George’s mother has likely passed on a toxic load and compromised gut flora to her baby, as she herself suffered from candidiasis at a young age. She also suffered from anxiety during her adolescence. Furthermore, George was born through C-section and was only breastfed for six months. This did not give George’s body the necessary tools to develop a strong immune system (Ip et al., 2007; Le Huërou-Luron, Blat, & Boudry, 2010; Neu & Rushing, 2011; Tannock et al., 2013).
The relationship between gut dysbiosis and PANDAS
PANDAS has been defined as an autoimmune disease (Swedo et al., 1998), and the gut microbiome is increasingly being considered crucial in development of autoimmunity (Belkaid & Hand, 2014; Cénit et al., 2014).
As suggested by Dr Campbell-McBride through her GAPS Nutritional Protocol, the source of autoimmune issues is gut dysbiosis, which damages the gut wall, causes abnormal digestion and absorption of food, and generates a flow of toxicity, while the body is unable to handle toxins from the environment (please see our companion article GAPS Nutritional Protocol: how healing the gut removes the basis for all chronic diseases, for further details). The immune system responds to all of these threats. Abnormal blood changes, which George displayed, were a consequence of his body reacting to undigested foods and toxins absorbing through the damaged gut wall (Nanagas & Kovalszki, 2019; Talley, 2008).
The effects of this toxicity on the brain lead to an ‘abnormality in processing sensory input’ (Campbell-McBride, 2010, p.338), commonly referred to as sensory processing disorder (SPD) in the autistic population (Clayton et al., 2018)
All GAPS people – people whose health issues stem from their damaged gut – experience SPD. In fact, many of George’s symptoms were the immediate result of an SPD: his phobias, his hypersensitivity to noise and odours, his restricted eating behaviours, and his attention deficit. With the GAPS Nutritional Protocol these symptoms of SPD disappeared.
Sleep disturbances are a dominant symptom of PANDAS and George was particularly prone to these. In their recent study, Smith et al. (2019) suggested that a more diverse microbiome is associated with better quality of sleep. Since young people diagnosed with PANDAS appear to have less biodiverse gut flora than their peers (Quagliarello et al., 2018), gut health could explain this aspect of PANDAS. In addition, Quagliarello et al. (2018) found that tyrosine levels were particularly low in their PANDAS sample. Tyrosine is a precursor of dopamine, which plays an important role in circadian rhythms (Korshunov, Blakemore, & Trombley, 2017).
George’s anxiety and psychotic symptoms significantly dropped once his microbiome found a better balance and once his body was nourished with nutrient-dense food. This resonates with past research suggesting gut health and mental sanity are intrinsically linked (Cade et al., 1990; Calabrese, Rapport, & Shelton, 1999; Dohan, 1966; Dohan & Grasberger, 1973; Hoffer, 1971; Kaplan, Crawford, Field, & Simpson, 2007; Singh, 1976; Torrey et al., 1984)
Contributions
Although widely used by patients around the world with promising results, the GAPS Nutritional Protocol has been scarcely studied in academic scientific literature. Among the few studies which have looked at the GAPS Nutritional Protocol (Abele, Tzivian, Meija, & Folkmanis, 2019; Babinska et al., 2020; Cekici & Sanlier, 2019; Çikili, Deniz, & Çakal, 2019; Nazarenkov et al., 2018), no research has explored it as a treatment for autoimmune diseases such as PANDAS.
We believe our case study fills recent research’s stated gaps when it comes to understanding PANDAS (Dale et al., 2006; Gamucci et al., 2019; Leckman et al., 2011; Orefici et al., 2016; Sigra et al., 2018).
Further Research
Given the lack of studies evaluating the effectiveness of the GAPS Nutritional Protocol treatment, we highly encourage researchers interested in addressing gut dysbiosis as the cause of psychological and/or physiological disorders to use the GAPS Nutritional Protocol in their practice and/or empirical studies.
Conclusion
The current study aimed to explore the GAPS Nutritional Protocol as a treatment for PANDAS. After nine months on the GAPS Diet, George’s symptoms had significantly improved. After 18 months, they had disappeared, to the point where his diagnosis was officially removed by medical authorities and all medications were stopped. The healing was also visible in important blood analyses such as mean platelet volume, neutrophil count, lymphocyte count, and eosinophil count, which despite being significantly out of range for multiple years, all returned to normal values after George followed the GAPS Nutritional Protocol. This case study suggests gut dysbiosis could be at the centre of PANDAS’ aetiology and that gut health should be given more consideration when it comes to treating autoimmune diseases.
Declarations
Ethics Approval and Consent to Participate
Not applicable. The study is retrospective. The patient engaged in the GAPS Nutritional Protocol willingly, i.e., they were not prompted by researchers for the purpose of the article. For privacy reasons, George is a pseudonym.
Consent for Publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Dr Natasha Campbell-McBride is the creator of the GAPS concept and the GAPS Nutritional Protocol.
Funding
No financial support has been received for the work reported.
Authors’ Contributions
Shantih Coro collected the data. Sophie Delaunay-Vagliasindi collected additional qualitative data, analysed all data, and wrote the manuscript. Natasha Campbell-McBride and Stephanie Seneff supervised and edited. All authors read and approved the final manuscript.
Acknowledgements
This case was written following the CARE guidelines.
Author’s Information
Sophie Delaunay-Vagliasindi holds a MSc degree in Developmental Psychology from the University of Kent, UK. She is a certified GAPS Coach. She is specialising in the impact of gut flora on development and conducting research for non-profit organizations, while studying for her second MSc degree in Clinical Psychology. Stephanie Seneff is a Senior Research Scientist at MIT in Cambridge, MA. She holds a B.S. degree from MIT in biology, and a Ph.D. in electrical engineering and computer science also from MIT. Her recent research interests are on the role of nutritional deficiencies and toxic chemicals in disease, with a focus on the mineral sulfur and the herbicide glyphosate. Shantih Coro is a Registered Dietician and experienced GAPS Practitioner. He holds a degree in Human Nutrition from the University of North Florida. He is currently specialising in Functional Medicine at the Institute for Functional Medicine and Functional Immunology with Dr. Samuel F. Yanuck. Natasha Campbell-McBride is a medical doctor with two postgraduate degrees: MMedSci (neurology), MMedSci (human nutrition). She is the creator of the GAPS concept and the GAPS Nutritional Protocol.
References
Abele S, Tzivian L, Meija L & Folkmanis V (2019) Low Carbohydrate Diet (SCD/GAPS) for Children with Autistic Spectrum Disorder. International society for nutritional psychiatry research (ISNPR) conference, London, United Kingdom, 20-22 October 2019.
Akhtari M, Curtis B & Waller EK (2009) Autoimmune neutropenia in adults. Autoimmunity reviews, 9(1), 62-66. https://doi.org/10.1016/j.autrev.2009.03.006.
Ates S, Oksuz H, Dogu B, Bozkus F, Ucmak H & Yanıt F (2015) Can mean platelet volume and mean platelet volume/platelet count ratio be used as a diagnostic marker for sepsis and systemic inflammatory response syndrome? Saudi medical journal, 36(10), 1186. https://doi.org/10.15537/smj.2015.10.10718.
Aydemir H, Piskin N, Akduman D, Kokturk F & Aktas E (2015) Platelet and mean platelet volume kinetics in adult patients with sepsis. Platelets, 26(4), 331-335. https://doi.org/10.3109/09537104.2012.701027.
Babinska K, Celusakova H, Belica I, Szapuova Z, Waczulikova I, Nemcsicsova D & Ostatnikova D (2020) Gastrointestinal symptoms and feeding problems and their associations with dietary interventions, food supplement use, and behavioral characteristics in a sample of children and adolescents with autism spectrum disorders. International Journal of Environmental Research and Public Health, 17(17), 6372. https://doi.org/10.3390/ijerph17176372.
Belkaid Y & Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell, 157(1), 121-141. https://doi.org/10.1016/j.cell.2014.03.011.
Boxer LA, Greenberg MS, Boxer GJ & Stossel TP (1975) Autoimmune neutropenia. New England Journal of Medicine, 293(15), 748-753. https://doi.org/10.1056/NEJM197510092931505.
Boxer LA (2012) How to approach neutropenia. Hematology 2010, the American Society of Hematology Education Program Book, 2012(1), 174-182. https://doi.org/10.1182/asheducation.V2012.1.174.3798251.
Bruin MC, von dem Borne, AEK, Tamminga RY, Kleijer M, Buddelmeijer L & de Haas M (1999) Neutrophil antibody specificity in different types of childhood autoimmune neutropenia. Blood, The Journal of the American Society of Hematology, 94(5), 1797-1802. https://doi.org/10.1182/blood.V94.5.1797.
Cade R, Wagemaker H, Privette RM, Fregly MJ, Rogers J & Orlando J (1990) The effect of dialysis and diet on schizophrenia. Psychiatry: A world perspective, 3(900), 494-500.
Campbell-McBride N (2010) Gut and psychology syndrome: natural treatment for autism, dyspraxia, ADD, dyslexia, ADHD, depression, schizophrenia. Chelsea Green Publishing.
Campbell-McBride N (2020) Gut and Physiology Syndrome: Natural Treatment for Allergies, Autoimmune Illness, Arthritis, Gut Problems, Fatigue, Hormonal Problems, Neurological Disease and More. Chelsea Green Publishing.
Calabrese JR, Rapport DJ & Shelton MD (1999) Fish oils and bipolar disorder: a promising but untested treatment. Archives of General Psychiatry, 56(5), 413-414. https://doi.org/10.1001/archpsyc.56.5.413.
Calaprice D, Tona J & Murphy TK (2018) Treatment of pediatric acute-onset neuropsychiatric disorder in a large survey population. Journal of child and adolescent psychopharmacology, 28(2), 92-103. https://doi.org/10.1089/cap.2017.0101.
Castoldi G, Liso V & Aversa F (2014) Ematologia. McGraw-Hill Education.
Cénit MC, Matzaraki V, Tigchelaar EF & Zhernakova A (2014) Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1842(10), 1981-1992. https://doi.org/10.1016/j.bbadis.2014.05.023.
Cekici H & Sanlier N (2019) Current nutritional approaches in managing autism spectrum disorder: A review. Nutritional neuroscience, 22(3), 145-155. https://doi.org/10.1080/1028415X.2017.1358481.
Çikili Y, Deniz S & Çakal B. Analysis Of The Effect Of GAPS Diet On Individuals With Autism Spectrum Disorder. Journal of Ahmet Kelesoglu Education Faculty, 1(1), 1-11.
Clayton G, Carrera HA, Martin ER, Morrison D & Bawazir AA (2018) A Biomedical Approach Via Telemedicine in the Treatment of a Child With Sensory Processing Disorder Using Diet and High-dose Biotin Intervention: A Case Report. Integrative Medicine: A Clinician’s Journal, 17(4), 52.
Cryan JF, O’Riordan KJ, Cowan CS, Sandhu KV, Bastiaanssen TF, Boehme M & Dinan TG (2019) The microbiota-gut-brain axis. Physiological reviews. https://doi.org/10.1152/physrev.00018.2018.
Dale RC, Church AJ, Candler PM, Chapman M, Martino D & Giovannoni G (2006) Serum autoantibodies do not differentiate PANDAS and Tourette syndrome from controls. Neurology, 66(10), 1612-1612. https://doi.org/10.1212/01.wnl.0000226832.36908.4c.
Diny NL, Rose NR & Čiháková D (2017) Eosinophils in autoimmune diseases. Frontiers in immunology, 8, 484. https://doi.org/10.3389/fimmu.2017.00484.
Dohan FC (1966) Cereals and schizophrenia data and hypothesis. Acta Psychiatrica Scandinavica, 42(2), 125-152. https://doi.org/10.1111/j.1600-0447.1966.tb01920.x.
Dohan FC & Grasberger JC (1973) Relapsed schizophrenics: earlier discharge from the hospital after cereal-free, milk-free diet. American Journal of Psychiatry, 130(6), 685-688. https://doi.org/10.1176/ajp.130.6.685.
Dursun A, Ozsoylu S & Akyildiz BN (2018) Neutrophil-to-lymphocyte ratio and mean platelet volume can be useful markers to predict sepsis in children. Pakistan journal of medical sciences, 34(4), 918. https://doi.org/10.12669/pjms.344.14547.
Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC & De Kock M (2017) What is the normal value of the neutrophil-to-lymphocyte ratio?. BMC research notes, 10(1), 1-4. https://doi.org/10.1186/s13104-016-2335-5.
Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K & Chang K (2015) Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: Presenting characteristics of the first 47 consecutive patients. Journal of child and adolescent psychopharmacology, 25(1), 38-47. https://doi.org/10.1089/cap.2014.0081.
Gamucci A, Uccella S, Sciarretta L, D’Apruzzo M, Calevo MG, Mancardi MM & De Grandis E (2019) PANDAS and PANS: clinical, neuropsychological, and biological characterization of a monocentric series of patients and proposal for a diagnostic protocol. Journal of child and adolescent psychopharmacology, 29(4), 305-312. https://doi.org/10.1089/cap.2018.0087.
Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL & Swedo SE (1999) A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biological psychiatry, 45(12), 1564-1571. https://doi.org/10.1016/S0006-3223(99)00020-7.
Gottschall EG (1994) Breaking the vicious cycle: intestinal health through diet. Ontario, Canada: Kirkton Press.
Haas SV & Haas MP (1951) Management of Celiac Disease. J. B.
Hamad H & Mangla A (2019) Lymphocytosis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549819/.
Hoffer A (1971) Megavitamin B-3 therapy for schizophrenia. Canadian Psychiatric Association Journal, 16(6), 499-504. https://doi.org/10.1177/070674377101600605.
Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D & Lau, J (2007) Breastfeeding and maternal and infant health outcomes in developed countries. Evidence report/technology assessment, (153), 1-186.
Kaplan BJ, Crawford SG, Field CJ & Simpson JSA (2007) Vitamins, minerals, and mood. Psychological bulletin, 133(5), 747. https://doi.org/10.1037/0033-2909.133.5.747.
Kawikova I, Grady BP, Tobiasova Z, Zhang Y, Vojdani A, Katsovich L & Leckman JF (2010) Children with Tourette’s syndrome may suffer immunoglobulin A dysgammaglobulinemia: preliminary report. Biological psychiatry, 67(7), 679-683. https://doi.org/10.1016/j.biopsych.2009.09.034.
Kolaczkowska E & Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nature reviews immunology, 13(3), 159-175. https://doi.org/10.1038/nri3399.
Korshunov KS, Blakemore LJ & Trombley PQ (2017) Dopamine: a modulator of circadian rhythms in the central nervous system. Frontiers in cellular neuroscience, 11, 91. https://doi.org/10.3389/fncel.2017.00091.
Kovalszki A & Weller PF (2016) Eosinophilia. Primary care, 43(4), 607. https://doi.org/10.1016/j.pop.2016.07.010.
Leckman JF, King RA, Gilbert DL, Coffey BJ, Singer HS, Dure IV LS & Kaplan EL (2011) Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. Journal of the American Academy of Child & Adolescent Psychiatry, 50(2), 108-118. https://doi.org/10.1016/j.jaac.2010.10.011.
Le Huërou-Luron I, Blat S & Boudry G (2010) Breast-v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutrition research reviews, 23(1), 23-36. https://doi.org/10.1017/S0954422410000065.
Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB & Huttenhower C (2017) Strains, functions and dynamics in the expanded Human Microbiome Project. Nature, 550(7674), 61-66. https://doi.org/10.1038/nature23889.
Lopetuso LR, Scaldaferri F, Petito V & Gasbarrini A (2013) Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut pathogens, 5(1), 1-8. https://doi.org/10.1186/1757-4749-5-23.
Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G & Gasbarrini A (2016) Gut microbiota in autism and mood disorders. World journal of gastroenterology, 22(1), 361. https://doi.org/10.3748/wjg.v22.i1.361.
Murphy TK, Brennan EM, Johnco C, Parker-Athill EC, Miladinovic B, Storch A & Lewin AB (2017) A double-blind randomized placebo-controlled pilot study of azithromycin in youth with acute-onset obsessive–compulsive disorder. Journal of child and adolescent psychopharmacology, 27(7), 640-651. https://doi.org/10.1089/cap.2016.0190.
Nanagas VC & Kovalszki A (2019) Gastrointestinal manifestations of hypereosinophilic syndromes and mast cell disorders: a comprehensive review. Clinical reviews in allergy & immunology, 57(2), 194-212. https://doi.org/10.1007/s12016-018-8695-y.
Nazarenkov N, Beeken L, Seeger K, Ananthakrishnan A, Khalili H, Lewis JD & Konijeti GG (2018) Nutritional Adequacy of Popular Defined Diets for Inflammatory Bowel Disease: 736. Official journal of the American College of Gastroenterology ACG, 113, S415. https://doi.org/10.1093/ecco-jcc/jjx180.779.
Neu J & Rushing J (2011) Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clinics in perinatology, 38(2), 321-331. https://doi.org/10.1016/j.clp.2011.03.008.
Orefici G, Cardona F, Cox CJ & Cunningham MW (2016) Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK333433/.
Palm NW, De Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L & Flavell RA (2014) Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell, 158(5), 1000-1010. https://doi.org/10.1016/j.cell.2014.08.006.
Price WA & Nguyen T (2016) Nutrition and physical degeneration: a comparison of primitive and modern diets and their effects.
Quagliariello A, Del Chierico F, Russo A, Reddel S, Conte G, Lopetuso LR & Putignani L (2018) Gut microbiota profiling and gut–brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Frontiers in microbiology, 9, 675. https://doi.org/10.3389/fmicb.2018.00675
Schmoeller D, Picarelli MM, Paz Munhoz T, Poli de Figueiredo CE & Staub H (2017) Mean platelet volume and immature platelet fraction in autoimmune disorders. Frontiers in medicine, 4, 146. https://doi.org/10.3389/fmed.2017.00146.
Seeley R, Stephens T & Tate P (2008) Anatomy and physiology. (8th ed.). New York: McGraw- Hill.
Shaaban HA & Safwat N (2020) Mean platelet volume in preterm: a predictor of early onset neonatal sepsis. The Journal of Maternal-Fetal & Neonatal Medicine, 33(2), 206-211. https://doi.org/10.1080/14767058.2018.1488161.
Sigra S, Hesselmark E & Bejerot S (2018) Treatment of PANDAS and PANS: a systematic review. Neuroscience & Biobehavioral Reviews, 86, 51-65. https://doi.org/10.1016/j.neubiorev.2018.01.001.
Singh MM (1976) Wheat gluten as a pathogenic factor in schizophrenia. Science, 191(4225), 401-402. https://doi.org/10.1126/science.1246624.
Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ & Tartar JL (2019) Gut microbiome diversity is associated with sleep physiology in humans. PLoS One, 14(10), e0222394. https://doi.org/10.1371/journal.pone.0222394.
Soydinc S, Turkbeyler IH, Pehlivan Y, Soylu G, Goktepe MF, Bilici M & Onat AM (2014) Mean platelet volume seems to be a valuable marker in patients with systemic sclerosis. Inflammation, 37(1), 100-106. https://doi.org/10.1007/s10753-013-9716-x.
Starkebaum G (2002) Chronic neutropenia associated with autoimmune disease. Seminars In Hematology, 39(2), 121-127. https://doi.org/10.1053/shem.2002.31918.
Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S & Lougee L (1998) Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. American Journal of Psychiatry, 155(2), 264-271.
Talley NJ (2008) Gut eosinophilia in food allergy and systemic and autoimmune diseases. Gastroenterology Clinics of North America, 37(2), 307-332. https://doi.org/10.1016/j.gtc.2008.02.008.
Tannock GW, Lawley B, Munro K, Gowri Pathmanathan S, Zhou SJ, Makrides M & Hodgkinson AJ (2013) Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Applied and environmental microbiology, 79(9), 3040-3048. https://doi.org/10.1128/AEM.03910-12.
Thienemann M, Murphy T, Leckman J, Shaw R, Williams K, Kapphahn C & Swedo S (2017) Clinical management of pediatric acute-onset neuropsychiatric syndrome: part I—psychiatric and behavioral interventions. Journal of child and adolescent psychopharmacology, 27(7), 566-573. https://doi.org/10.1089/cap.2016.0145.
Torrey EF, McGuire M, O’Hare A, Walsh D & Spellman MP (1984) Endemic psychosis in western Ireland. The American journal of psychiatry. https://doi.org/10.1176/ajp.141.8.966.
Uzun AD, Sapmaz ŞY, Çakır B & Kandemir H (2020) Could neutrophil-to-lymphocyte ratio be an important parameter in children and adoles-cents with obsessive compulsive disorder?. Turkish J Clinical Psychiatry, 23, 101-105. https://doi.org/10.5505/kpd.2020.02359.
Velasquez-Manoff M (2015) The Peacekeepers. Nature, 518(7540), S3-S5. https://doi.org/10.1038/518S3a.
Wang Q, Lu C, Guo T & Qian J (2009) Eosinophilia Associated With Chronic Pancreatitis. Pancreas, 38(2), 149-153. https://doi.org/10.1097/MPA.0b013e31818d8ecc.
Wilbur C, Bitnun A, Kronenberg S, Laxer RM, Levy DM, Logan WJ & Yeh EA (2019) PANDAS/PANS in childhood: Controversies and evidence. Paediatrics & child health, 24(2), 85-91. https://doi.org/10.1093/pch/pxy145.
Yavuz S & Ece A (2014) Mean platelet volume as an indicator of disease activity in juvenile SLE. Clinical rheumatology, 33(5), 637-641. https://doi.org/10.1007/s10067-014-2540-3.
Yuri Gasparyan A, Ayvazyan L, P Mikhailidis D & D Kitas G (2011) Mean platelet volume: a link between thrombosis and inflammation?. Current pharmaceutical design, 17(1), 47-58. https://doi.org/10.2174/138161211795049804


